Quality of Life, Anxiety, and Depression in Patients With Early-Stage Mycosis Fungoides and the Effect of Oral Psoralen Plus UV-A (PUVA) Photochemotherapy on it
- PMID: 32850876
- PMCID: PMC7419471
- DOI: 10.3389/fmed.2020.00330
Quality of Life, Anxiety, and Depression in Patients With Early-Stage Mycosis Fungoides and the Effect of Oral Psoralen Plus UV-A (PUVA) Photochemotherapy on it
Abstract
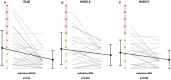
Background: Little is known about psychological discomfort and quality of life (QoL) in early stage mycosis fungoides (MF) and the effect of psoralen plus UV-A (PUVA) on it. Objective: To evaluate QoL, anxiety, and depression with validated instruments in early stage MF patients and whether PUVA treatment improves it. Methods: Patients with stage IA to IIA MF were treated with PUVA twice weekly for 12-24 weeks, followed by maintenance treatment or not, in a prospective randomized clinical trial. Patients completed a questionnaire on DLQI as well as the Hospital Anxiety and Depression Scale (HADS) prior to therapy, after their last PUVA exposure, and after the PUVA maintenance or observance phase. Results: For 24 patients with early stage MF, completed questionnaires were available and analyzed. Prior to treatment, 17% reported strong (DLQI > 10) and 29% moderate impairment (DLQI 6-10) in QoL; 33% of patients reported HADS scores indicating anxiety, and 21% reported scores indicating depression. PUVA significantly improved overall QoL by reducing mean DLQI scores by 58.6% (p = 0.003), HADS-A by 30% (p = 0.045), and HADS-D by 44% (p = 0.002). Improvements in QoL and psychological well-being seemed to be sustained, irrespective of maintenance treatment or not. Limitations: Small sample size. Conclusions: PUVA sustainably improves QoL and psychological well-being in patients with early stage MF. Clinical trial registration: ClinicalTrials.gov identifier: NCT01686594.
Keywords: PUVA; anxiety; depression; mycosis fungoides; phototherapy; quality of life.
Copyright © 2020 Graier, Fink-Puches, Porkert, Lang, Pöchlauer, Ratzinger, Tanew, Selhofer, Sator, Hofer, Gruber-Wackernagel, Legat, Vieyra-Garcia, Quehenberger and Wolf.
Figures

References
-
- Molloy K, Jonak C, Woei-A-Jin FJSH, Guenova E, Busschots AM, Bervoets A, et al. . Characteristics associated with significantly worse quality of life in mycosis fungoides/Sézary syndrome from the prospective cutaneous lymphoma International prognostic index (PROCLIPI) study. Br J Dermatol. (2019) 182–770–9. 10.1111/bjd.18089 - DOI - PubMed
-
- Herbosa CM, Semenov YR, Rosenberg AR, Mehta-Shah N, Musiek AC. Clinical severity measures and quality of life burden in patients with mycosis fungoides and Sézary syndrome: comparison of generic and dermatology-specific instruments. J Eur Acad Dermatology Venereol. (2019) 34:995–1003. 10.1111/jdv.16021 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

