A General Mechanism of Green-to-Red Photoconversions of GFP
- PMID: 32850965
- PMCID: PMC7405548
- DOI: 10.3389/fmolb.2020.00176
A General Mechanism of Green-to-Red Photoconversions of GFP
Abstract
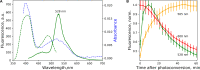
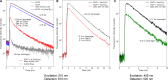
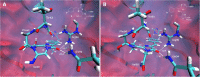
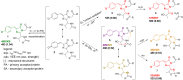
Here we dissect the phenomena of oxidative and reductive green-to-red photoconversion of the Green Fluorescent Protein. We characterize distinct orange- and red-emitting forms (λabs/λem = 490/565 nm; λabs/λem = 535/600 nm) arising during the Enhanced Green Fluorescent Protein (EGFP) photoconversion under low-oxygen conditions in the presence of reductants. These forms spectroscopically differ from that observed previously in oxidative redding (λabs/λem = 575/607 nm). We also report on a new green-emitting state (λabs/λem = 405/525 nm), which is formed upon photoconversion under the low-oxygen conditions. Based on the spectral properties of these forms, their light-independent time evolution, and the high-level computational studies, we provide a structural basis for various photoproducts. Under the low-oxygen conditions, the neutral quinoid-like structure formed via a two-electron oxidation process is found to be a key intermediate and a most likely candidate for the novel green-emitting state of the chromophore. The observed large Stokes shift is traced to the formation of the zwitterionic form of the chromophore in the excited state. Subsequently, this form undergoes two types of cyclization reactions, resulting in the formation of either the orange-emitting state (λabs/λem = 490/565 nm) or the red-emitting form (λabs/λem = 535/600 nm). The T65G mutant lacks one of the proposed cyclization pathways and, indeed, the photoconverted T65G EGFP exhibits a single orange-emitting state. In oxidative redding, the red-emitting state resembles the structure of the chromophore from asFP595 (λabs/λem = 572/595 nm), which is directly formed upon two-electron oxidation and deprotonation bypassing the formation of the quinoid-like structure. Our results disclose a general "oxidative" mechanism of various green-to-red photoconversions of EGFP, providing a link between oxidative redding and the photoconversion under low-oxygen conditions.
Keywords: EGFP; oxidative redding; photoconversion; photoinduced electron transfer; reductive redding.
Copyright © 2020 Gorbachev, Petrusevich, Kabylda, Maksimov, Lukyanov, Bogdanov, Baranov, Bochenkova and Mishin.
Figures




References
-
- Bochenkova A. V., Andersen L. H. (2013). Ultrafast dual photoresponse of isolated biological chromophores: link to the photoinduced mode-specific non-adiabatic dynamics in proteins. Faraday Discuss 163 297–319. discussion 393–432. - PubMed
-
- Bogdanov A. M., Acharya A., Titelmayer A. V., Mamontova A. V., Bravaya K. B., Kolomeisky A. B., et al. (2016). Turning on and off photoinduced electron transfer in fluorescent proteins by π-stacking. Halide Binding, and Tyr145 Mutations. J. Am. Chem. Soc. 138 4807–4817. 10.1021/jacs.6b00092 - DOI - PubMed
LinkOut - more resources
Full Text Sources

