Geographical Variations in Virulence Factors and Antimicrobial Resistance Amongst Staphylococci Isolated From Dogs From the United Kingdom and Romania
- PMID: 32851008
- PMCID: PMC7396606
- DOI: 10.3389/fvets.2020.00414
Geographical Variations in Virulence Factors and Antimicrobial Resistance Amongst Staphylococci Isolated From Dogs From the United Kingdom and Romania
Abstract
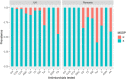
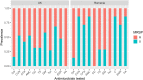
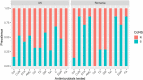
The objective of this study was to compare virulence and resistance factors of mucosal and cutaneous staphylococci from dogs with pyoderma in the UK and Romania, two countries with different approaches to antimicrobial use in companion animals. Staphylococcal isolates (n = 166) identified to the species level as being Staphylococcus pseudintermedius or coagulase negative (CoNS) were analyzed for their antimicrobial resistance (AMR) profile and presence of resistance and virulence genes. Of the investigated isolates, 26 were methicillin-resistant S. pseudintermedius (MRSP), 89 were methicillin-susceptible S. pseudintermedius (MSSP) and 51 were coagulase negative staphylococci (CoNS). A significantly larger number of isolates originating from Romania were resistant to clindamycin, tetracycline, and chloramphenicol compared to the UK isolates (P < 0.05). Resistance to amoxicillin-clavulanic acid, gentamicin, and trimethoprim-sulphamethoxazole was more evident in UK isolates. Fusidic acid resistance was common in Staphylococcus spp. isolates from both countries. Most isolates carried virulence factors associated with siet (exfoliative toxin) and luk (leucocidin) genes. All MRSP UK isolates exhibited fusidic acid resistance genes whilst this was very rare in the MRSP isolates from Romania. The chlorhexidine resistance gene qacA/B was frequently identified in CoNS isolates from the UK (P < 0.001). The current study documented differences in antimicrobial resistance profiles of Staphylococcus spp. isolates from dogs in two geographical locations in Europe, which could reflect differences in antimicrobial prescribing patterns. The study also highlights the need for further studies and interventions on antimicrobial use, prescribing patterns and AMR surveillance in companion animals in Romania.
Keywords: Romania; Staphylococcus pseudintermedius; UK; antimicrobial resistance; dog; pyoderma.
Copyright © 2020 Hritcu, Schmidt, Salem, Maciuca, Moraru, Lipovan, Mareş, Solcan and Timofte.
Figures
References
-
- Scott DW, Miller WH, Griffin CE. Muller and Kirk's Small Animal Dermatology. 6th ed Philadelphia, PA: WB Saunders; (2000).
-
- Gómez Sanz E. Staphylococcus aureus and Staphylococcus pseudintermedius in animals: molecular epidemiology, antimicrobial resistance, virulence and zoonotic potential [Doctoral thesis]. Universidad de la Rioja, Logroño, Spain: (2013).
LinkOut - more resources
Full Text Sources
Miscellaneous

