Pharmacokinetics of Pimobendan and Its Metabolite O-Desmethyl-Pimobendan Following Rectal Administration to Healthy Dogs
- PMID: 32851013
- PMCID: PMC7417621
- DOI: 10.3389/fvets.2020.00423
Pharmacokinetics of Pimobendan and Its Metabolite O-Desmethyl-Pimobendan Following Rectal Administration to Healthy Dogs
Abstract
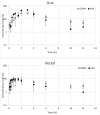
Objective: This study describes the pharmacokinetics of parent pimobendan (PIM) and its active metabolite, o-desmethyl-pimobendan (ODMP), after oral and rectal administration of pimobendan to healthy dogs. Animals: A total of eight healthy privately owned dogs were used in this study. Procedures: The dogs received a single dose (0.5 mg/kg) of a commercially available pimobendan tablet per os (PO). Twelve blood samples were collected over a 12-h period for pharmacokinetic analysis. After a 24-h washout period, the dogs received the same dose of pimobendan solution per rectum (PR), and samples were obtained at the same time for analysis. Results: For PIM, PO vs. PR, respectively, the mean maximum plasma concentration (C max, ng/ml) was 49.1 ± 28.7 vs. 10.1 ± 2, the time to reach a maximum concentration (T max, h) was 2.1 ± 0.9 vs. 1 ± 0.4, the disappearance half-life (t 1/2, h) was 1.8 ± 0.8 vs. 2.2 ± 0.6, and the area under the concentration-time curve (AUC, ng*h/ml) was 148.4 ± 71.6 vs. 31.1 ± 11.9, with relative bioavailability (F, %) of 25 ± 8. For ODMP, PO vs. PR, respectively, C max was 30.9 ± 10.4 vs. 8.8 ± 4.8, T max was 3.2 ± 1.6 vs. 1.7 ± 1.1, and t 1/2 was 5.0 ± 2.7 vs. 8.3 ± 4.8, with AUC of 167.8 ± 36.2 vs. 50.1 ± 19.2 and F of 28 ± 6. The differences between PO and PR were significant (P < 0.03) for AUC and C max for both PIM and ODMP. Conclusions and Clinical Relevance: The pharmacokinetics of PIM and ODMP were described following PO and PR administration. The findings suggest that pimobendan PR might achieve effective concentrations and, as such, warrant future studies of clinical effectiveness in treating dogs with congestive heart failure and which are unable to receive medication PO.
Keywords: bioavailability; congestive heart failure; pharmacokinetics; pimobendan; rectal drug administration.
Copyright © 2020 Her, Kuo, Winter, Cruz-Espindola, Bacek and Boothe.
Figures
Similar articles
-
Pharmacodynamics and Pharmacokinetics of Injectable Pimobendan and Its Metabolite, O-Desmethyl-Pimobendan, in Healthy Dogs.Front Vet Sci. 2021 Aug 20;8:656902. doi: 10.3389/fvets.2021.656902. eCollection 2021. Front Vet Sci. 2021. PMID: 34490386 Free PMC article.
-
Pharmacokinetics of pimobendan after oral administration to dogs with myxomatous mitral valve disease.J Vet Intern Med. 2023 Nov-Dec;37(6):2003-2010. doi: 10.1111/jvim.16891. Epub 2023 Sep 30. J Vet Intern Med. 2023. PMID: 37776546 Free PMC article.
-
Single-dose pharmacokinetics and cardiovascular effects of oral pimobendan in healthy cats.J Vet Cardiol. 2016 Dec;18(4):310-325. doi: 10.1016/j.jvc.2016.07.001. Epub 2016 Sep 6. J Vet Cardiol. 2016. PMID: 27613648 Clinical Trial.
-
Effects of aprepitant on the pharmacokinetics of ondansetron and granisetron in healthy subjects.Clin Ther. 2003 May;25(5):1407-19. doi: 10.1016/s0149-2918(03)80128-5. Clin Ther. 2003. PMID: 12867217 Review.
-
Calcium sensitization with pimobendan: pharmacology, haemodynamic improvement, and sudden death in patients with chronic congestive heart failure.Eur Heart J. 1993 Apr;14(4):551-66. doi: 10.1093/eurheartj/14.4.551. Eur Heart J. 1993. PMID: 8472722 Review.
Cited by
-
Hemodynamic effect of pimobendan following intramuscular and intravenous administration in healthy dogs: A pilot study.Front Vet Sci. 2022 Oct 12;9:969304. doi: 10.3389/fvets.2022.969304. eCollection 2022. Front Vet Sci. 2022. PMID: 36311676 Free PMC article.
-
Preliminary Bioequivalence of an Oral Pimobendan Solution Formulation with Reference Solution Formulation in Beagle Dogs.Vet Sci. 2022 Mar 17;9(3):141. doi: 10.3390/vetsci9030141. Vet Sci. 2022. PMID: 35324869 Free PMC article.
-
Pharmacodynamics and Pharmacokinetics of Injectable Pimobendan and Its Metabolite, O-Desmethyl-Pimobendan, in Healthy Dogs.Front Vet Sci. 2021 Aug 20;8:656902. doi: 10.3389/fvets.2021.656902. eCollection 2021. Front Vet Sci. 2021. PMID: 34490386 Free PMC article.
-
No impact of polymorphism in the phosphodiesterase 5A gene in Cavalier King Charles Spaniels on pimobendan-induced inhibition of platelet aggregation response.J Vet Intern Med. 2023 Nov-Dec;37(6):2145-2156. doi: 10.1111/jvim.16871. Epub 2023 Sep 24. J Vet Intern Med. 2023. PMID: 37743723 Free PMC article.
-
Pharmacokinetics of pimobendan after oral administration to dogs with myxomatous mitral valve disease.J Vet Intern Med. 2023 Nov-Dec;37(6):2003-2010. doi: 10.1111/jvim.16891. Epub 2023 Sep 30. J Vet Intern Med. 2023. PMID: 37776546 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials