In vitro Effects of Methylprednisolone Acetate on Equine Deep Digital Flexor Tendon-Derived Cells
- PMID: 32851046
- PMCID: PMC7419577
- DOI: 10.3389/fvets.2020.00486
In vitro Effects of Methylprednisolone Acetate on Equine Deep Digital Flexor Tendon-Derived Cells
Abstract
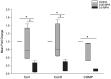
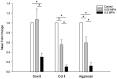
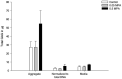
Primary deep digital flexor tendon (DDFT) pathologies and those accompanying degenerative changes of navicular bone fibrocartilage are major causes of lameness associated with navicular disease. Intrasynovial corticosteroids are mainstay in the treatment due to the anti-inflammatory effects, but their effect on DDFT cell biosynthesis are unknown. The objective of this in-vitro study was to investigate the effects of methylprednisolone acetate (MPA) on cells isolated from the dorsal fibrocartilaginous region of forelimb DDFTs (DDFT-derived cells) of 5 horses (aged 11-17 years). Non-adherent aggregate cultures were established from third passage cells over a 72 to 96-h duration prior to treating with medium containing 0 (control), 0.05 and 0.5 mg/mL MPA for 24 h. Tendon and cartilage extracellular matrix (ECM) related gene expression, cell aggregate and culture medium GAG contents, culture medium collagen and MMP-3 and-13 concentrations were measured. After 24 h of treatment, only the higher MPA concentration (0.5 mg/mL) significantly down-regulated tendon ECM related genes; whereas, both MPA doses significantly down-regulated cartilage ECM related genes. MPA treatment did not affect the total GAG content of DDFT-derived cells or total GAG, soluble collagen and MMP-3 and-13 contents in culture medium compared to untreated controls. Future studies to determine the response of DDFT-derived cells with longer exposure times to corticosteroids and in the presence of inflammatory cytokines are necessary. These results are a first step in assessing the effects of intrasynovial medications on equine DDFT, for which currently no information exists.
Keywords: ECM mRNA expression; GAG; MMP-3/-13; collagen; deep digital flexor tendon; horse; methylprednisolone acetate; navicular disease.
Copyright © 2020 Sullivan, Altmann, Brokken and Durgam.
Figures

References
-
- Marsh CA, Schneider RK, Sampson SN, Roberts GD. Response to injection of the navicular bursa with corticosteroid and hyaluronan following high-field magnetic resonance imaging in horses with signs of navicular syndrome: 101 cases (2000-2008). J Am Vet Med Assoc. (2012) 241:1353–64. 10.2460/javma.241.10.1353 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

