EED-mediated histone methylation is critical for CNS myelination and remyelination by inhibiting WNT, BMP, and senescence pathways
- PMID: 32851157
- PMCID: PMC7423366
- DOI: 10.1126/sciadv.aaz6477
EED-mediated histone methylation is critical for CNS myelination and remyelination by inhibiting WNT, BMP, and senescence pathways
Abstract
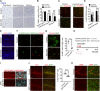
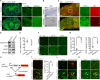
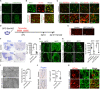
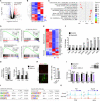
Mutations in the polycomb repressive complex 2 (PRC2) can cause Weaver-like syndrome, wherein a patient cohort exhibits abnormal white matter; however, PRC2 functions in CNS myelination and regeneration remain elusive. We show here that H3K27me3, the PRC2 catalytic product, increases during oligodendrocyte maturation. Depletion of embryonic ectoderm development (EED), a core PRC2 subunit, reduces differentiation of oligodendrocyte progenitors (OPCs), and causes an OPC-to-astrocyte fate switch in a region-specific manner. Although dispensable for myelin maintenance, EED is critical for oligodendrocyte remyelination. Genomic occupancy and transcriptomic analyses indicate that EED establishes a chromatin landscape that selectively represses inhibitory WNT and bone morphogenetic protein (BMP) signaling, and senescence-associated programs. Blocking WNT or BMP pathways partially restores differentiation defects in EED-deficient OPCs. Thus, our findings reveal that EED/PRC2 is a crucial epigenetic programmer of CNS myelination and repair, while demonstrating a spatiotemporal-specific role of PRC2-mediated chromatin silencing in shaping oligodendrocyte identity and lineage plasticity.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Laugesen A., Helin K., Chromatin repressive complexes in stem cells, development, and cancer. Cell Stem Cell 14, 735–751 (2014). - PubMed
-
- Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., Jones R. S., Zhang Y., Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 (2002). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

