Patient-reported outcomes from the investigational device exemption study of the Tablo hemodialysis system
- PMID: 32851807
- PMCID: PMC7692883
- DOI: 10.1111/hdi.12869
Patient-reported outcomes from the investigational device exemption study of the Tablo hemodialysis system
Abstract
Introduction: We recently completed an Investigational Device Exemption (IDE) study in which 30 patients were enrolled (13 patients previously on home hemodialysis (HHD) and 17 patients new to HHD) and treated with the Tablo Hemodialysis System (Outset Medical, Inc., San Jose, CA) for 8 weeks in-center and 8 weeks in-home with an interim 2-4 week transition period for home training.
Methods: In addition to assessments of urea kinetics, events related to safety, and operational issues (e.g., alarm resolution), we obtained data on several parameters of health-related quality of life, including time to recovery (TTR), the EQ-5D-5L (a well-validated measure of general health status), and the quality of sleep and related symptoms, to further assess the safety of HHD with Tablo. We compared results obtained during the in-center and in-home phases of the trial.
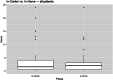
Results: Twenty-eight of 30 patients (93%) completed all trial periods. Adherence to the prescribed four treatments per week schedule was 96% in-center and 99% in-home. Median TTR was 1.5 hours (10th, 90th percentile range 0.17 to 12, mean TTR 3.68 ± 5.88 hours) during the in-center and 2 hours (10th, 90th percentile range 0 to 6.0, mean TTR 3.04 ± 5.14 hours) during the at-home phase (Wilcoxon signed rank p = 0.57). Median index values on the EQ-5D-5L were similar during the in-center (0.832, 10th, 90th percentile range 0.617 to 1, mean 0.817 ± 0.165) and in-home (0.826, 10th, 90th percentile range 0.603 to 1, mean 0.821 ± 0.163) trial phases (Wilcoxon signed rank p = 0.36). Patients reported feeling alert or well-rested with little difficulty falling or staying asleep or feeling tired and worn out when using Tablo in either environment.
Conclusion: When using Tablo in-home, patients reported similar TTR, general health status, and sleep quality and related symptoms compared to using Tablo in-center. (294 words).
Keywords: Clinical trial; health-related quality of life; home hemodialysis; time to recovery.
© 2020 Outset Medical. Hemodialysis International published by Wiley Periodicals LLC on behalf of International Society for Hemodialysis.
Figures


References
-
- Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R. Minutes to recovery after a hemodialysis session: A simple health‐related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol. 2006;1:952–959. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

