Interaction of SARS-CoV-2 and Other Coronavirus With ACE (Angiotensin-Converting Enzyme)-2 as Their Main Receptor: Therapeutic Implications
- PMID: 32851855
- PMCID: PMC7480804
- DOI: 10.1161/HYPERTENSIONAHA.120.15256
Interaction of SARS-CoV-2 and Other Coronavirus With ACE (Angiotensin-Converting Enzyme)-2 as Their Main Receptor: Therapeutic Implications
Abstract
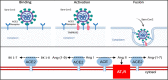
Severe acute respiratory syndrome coronavirus (SARS-CoV)-2 originated from Wuhan, China, in December 2019 and rapidly spread to other areas worldwide. Since then, coronavirus disease 2019 (COVID-19) has reached pandemic proportions with >570 000 deaths globally by mid-July 2020. The magnitude of the outbreak and the potentially severe clinical course of COVID-19 has led to a burst of scientific research on this novel coronavirus and its host receptor ACE (angiotensin-converting enzyme)-2. ACE2 is a homolog of the ACE that acts on several substrates in the renin-Ang (angiotensin) system. With unprecedented speed, scientific research has solved the structure of SARS-CoV-2 and imaged its binding with the ACE2 receptor. In SARS-CoV-2 infection, the viral S (spike) protein receptor-binding domain binds to ACE2 to enter the host cell. ACE2 expression in the lungs is relatively low, but it is present in type II pneumocytes-a cell type also endowed with TMPRSS2 (transmembrane protease serine 2). This protease is critical for priming the SARS-CoV-2 S protein to complex with ACE2 and enter the cells. Herein, we review the current understanding of the interaction of SARS-CoV-2 with ACE2 as it has rapidly unfolded over the last months. While it should not be assumed that we have a complete picture of SARS-CoV-2 mechanism of infection and its interaction with ACE2, much has been learned with clear therapeutic implications. Potential therapies aimed at intercepting SARS-CoV-2 from reaching the full-length membrane-bound ACE2 receptor using soluble ACE2 protein and other potential approaches are briefly discussed as well.
Keywords: China; coronavirus; coronavirus infections; renin-angiotensin system; serine.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

