Therapeutic Potential of Afatinib in NRG1 Fusion-Driven Solid Tumors: A Case Series
- PMID: 32852072
- PMCID: PMC7794194
- DOI: 10.1634/theoncologist.2020-0379
Therapeutic Potential of Afatinib in NRG1 Fusion-Driven Solid Tumors: A Case Series
Abstract
Background: Neuregulin 1 (NRG1) fusions, which activate ErbB signaling, are rare oncogenic drivers in multiple tumor types. Afatinib is a pan-ErbB family inhibitor that may be an effective treatment for NRG1 fusion-driven tumors.
Patients and methods: This report summarizes pertinent details, including best tumor response to treatment, for six patients with metastatic NRG1 fusion-positive tumors treated with afatinib.
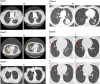
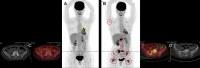
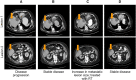
Results: The six cases include four female and two male patients who ranged in age from 34 to 69 years. Five of the cases are patients with lung cancer, including two patients with invasive mucinous adenocarcinoma and three patients with nonmucinous adenocarcinoma. The sixth case is a patient with colorectal cancer. NRG1 fusion partners for the patients with lung cancer were either CD74 or SDC4. The patient with colorectal cancer harbored a novel POMK-NRG1 fusion and a KRAS mutation. Two patients received afatinib as first- or second-line therapy, three patients received the drug as third- to fifth-line therapy, and one patient received afatinib as fifteenth-line therapy. Best response with afatinib was stable disease in two patients (duration up to 16 months when combined with local therapies) and partial response (PR) of >18 months in three patients, including one with ongoing PR after 27 months. The remaining patient had a PR of 5 months with afatinib 40 mg/day, then another 6 months after an increase to 50 mg/day.
Conclusion: This report reviews previously published metastatic NRG1 fusion-positive tumors treated with afatinib and summarizes six previously unpublished cases. The latter include several with a prolonged response to treatment (>18 months), as well as the first report of efficacy in NRG1 fusion-positive colorectal cancer. This adds to the growing body of evidence suggesting that afatinib can be effective in patients with NRG1 fusion-positive tumors.
Key points: NRG1 fusions activate ErbB signaling and have been identified as oncogenic drivers in multiple solid tumor types. Afatinib is a pan-ErbB family inhibitor authorized for the treatment of advanced non-small cell lung cancer that may be effective in NRG1 fusion-driven tumors. This report summarizes six previously unpublished cases of NRG1 fusion-driven cancers treated with afatinib, including five with metastatic lung cancer and one with metastatic colorectal cancer. Several patients showed a prolonged response of >18 months with afatinib treatment. This case series adds to the evidence suggesting a potential role for afatinib in this area of unmet medical need.
Keywords: Afatinib; Case series; ErbB-targeted treatment; Gene fusion; NRG1.
© AlphaMed Press 2020.
Conflict of interest statement
Figures

Comment in
-
Plain language summary of publication: new information for the potential role of afatinib in treating people with NRG1 gene fusion-positive cancer.Future Oncol. 2022 Jun;18(18):2193-2200. doi: 10.2217/fon-2021-0855. Epub 2022 Apr 11. Future Oncol. 2022. PMID: 35400204
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

