Immunogenicity of an adalimumab biosimilar, FKB327, and its reference product in patients with rheumatoid arthritis
- PMID: 32852139
- PMCID: PMC7754138
- DOI: 10.1111/1756-185X.13951
Immunogenicity of an adalimumab biosimilar, FKB327, and its reference product in patients with rheumatoid arthritis
Abstract
Aim: This study, FKB327-003, is a phase 3, open-label extension (OLE) study comparing the long-term immunogenicity of an adalimumab biosimilar, FKB327 (F), with the reference product (RP).
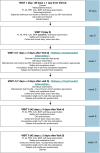
Methods: In the OLE, patients completing 24 weeks of an initial randomized, double-blind (DB) study (Period 1) with clinical response and no safety concerns were rerandomized to F or RP, so that two-thirds of patients remained on the same treatment and one-third switched to the alternate treatment for weeks 24 through 54 (OLE weeks 0-30; Period 2), then all received F through week 100 (OLE week 76; Period 3). Treatment sequences were F-F-F (no switch), RP-F-F and RP-RP-F (single switch), and F-RP-F (double switch). Patients who entered the OLE study were evaluated for immunogenicity across switching sequences.
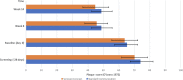
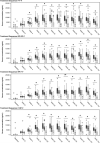
Results: The proportion of patients with positive antidrug antibody (ADA) status at the end of Period 1 was 61.7% and 60.0% for F and RP, respectively. The proportion of patients with positive ADA status did not increase throughout Period 1, and was similar for F and RP at all time points. At the end of Period 3, the proportion of patients with positive ADA status was lower in all treatment sequences, at 51.1%, 54.4%, 48.1%, and 42.5% for F-F-F, F-RP-F, RP-F-F, and RP-RP-F, respectively.
Conclusion: The RP and F showed comparable immunogenicity characteristics after long-term administration. Development of ADAs with the RP and F was similar, and was not impacted by switching and double switching between F and RP treatment.
Keywords: TNF inhibitors; adalimumab; biosimilars; immunogenicity; rheumatoid arthritis; switching.
© 2020 The Authors. International Journal of Rheumatic Diseases published by Asia Pacific League of Associations for Rheumatology and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Dr Alten has received consultant fees from Mylan Inc, and has been a paid speaker for Mylan Inc Dr Markland reports personal fees from Fujifilm Kyowa Kirin Biologics Co. Ltd. during the conduct of the study, as well as personal fees from Fujifilm Kyowa Kirin Biologics Co. Ltd. outside the submitted work. Dr Boyce has nothing to disclose. Mr Kawakami is an employee of Fujifilm Kyowa Kirin Biologics Co. Ltd. Dr Muniz is an employee and shareholder of Mylan Inc Dr Genovese has received consultant fees from Fujifilm Kyowa Kirin Biologics Co. Ltd., including for the design of this trial.
Figures
References
-
- US Food and Drug Administration . Biosimilar and interchangeable products. 2017. www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandAppr.... Accessed October 17, 2018
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

