Organic Photovoltaic Pseudocapacitors for Neurostimulation
- PMID: 32852189
- PMCID: PMC7582621
- DOI: 10.1021/acsami.0c11581
Organic Photovoltaic Pseudocapacitors for Neurostimulation
Abstract
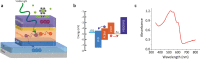
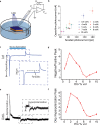
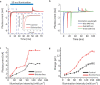
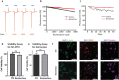
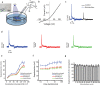
Neural interfaces are the fundamental tools to understand the brain and cure many nervous-system diseases. For proper interfacing, seamless integration, efficient and safe digital-to-biological signal transduction, and long operational lifetime are required. Here, we devised a wireless optoelectronic pseudocapacitor converting the optical energy to safe capacitive currents by dissociating the photogenerated excitons in the photovoltaic unit and effectively routing the holes to the supercapacitor electrode and the pseudocapacitive electrode-electrolyte interfacial layer of PEDOT:PSS for reversible faradic reactions. The biointerface showed high peak capacitive currents of ∼3 mA·cm-2 with total charge injection of ∼1 μC·cm-2 at responsivity of 30 mA·W-1, generating high photovoltages over 400 mV for the main eye photoreception colors of blue, green, and red. Moreover, modification of PEDOT:PSS controls the charging/discharging phases leading to rapid capacitive photoresponse of 50 μs and effective membrane depolarization at the single-cell level. The neural interface has a device lifetime of over 1.5 years in the aqueous environment and showed stability without significant performance decrease after sterilization steps. Our results demonstrate that adopting the pseudocapacitance phenomenon on organic photovoltaics paves an ultraefficient, safe, and robust way toward communicating with biological systems.
Keywords: PEDOT:PSS; bioelectronics; neurostimulation; organic photovoltaics; pseudocapacitors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Autoclave Sterilization of PEDOT:PSS Electrophysiology Devices.Adv Healthc Mater. 2016 Dec;5(24):3094-3098. doi: 10.1002/adhm.201600870. Epub 2016 Nov 25. Adv Healthc Mater. 2016. PMID: 27885829
-
A Wearable Supercapacitor Based on Conductive PEDOT:PSS-Coated Cloth and a Sweat Electrolyte.Adv Mater. 2020 Jun;32(24):e1907254. doi: 10.1002/adma.201907254. Epub 2020 May 11. Adv Mater. 2020. PMID: 32390218
-
Tissue-Like Optoelectronic Neural Interface Enabled by PEDOT:PSS Hydrogel for Cardiac and Neural Stimulation.Adv Healthc Mater. 2022 Apr;11(8):e2102160. doi: 10.1002/adhm.202102160. Epub 2022 Jan 12. Adv Healthc Mater. 2022. PMID: 34969168
-
Functional Sensing Interfaces of PEDOT:PSS Organic Electrochemical Transistors for Chemical and Biological Sensors: A Mini Review.Sensors (Basel). 2019 Jan 9;19(2):218. doi: 10.3390/s19020218. Sensors (Basel). 2019. PMID: 30634408 Free PMC article. Review.
-
Electrochemical polymerization of conducting polymers in living neural tissue.J Neural Eng. 2007 Jun;4(2):L6-L13. doi: 10.1088/1741-2560/4/2/L02. Epub 2007 Feb 2. J Neural Eng. 2007. PMID: 17409471 Review.
Cited by
-
Bioresorbable thin-film silicon diodes for the optoelectronic excitation and inhibition of neural activities.Nat Biomed Eng. 2023 Apr;7(4):486-498. doi: 10.1038/s41551-022-00931-0. Epub 2022 Sep 5. Nat Biomed Eng. 2023. PMID: 36065014
-
From innovation to clinic: Emerging strategies harnessing electrically conductive polymers to enhance electrically stimulated peripheral nerve repair.Mater Today Bio. 2024 Dec 19;30:101415. doi: 10.1016/j.mtbio.2024.101415. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39816667 Free PMC article.
-
MnO2 Nanoflower Integrated Optoelectronic Biointerfaces for Photostimulation of Neurons.Adv Sci (Weinh). 2023 Sep;10(25):e2301854. doi: 10.1002/advs.202301854. Epub 2023 Jun 29. Adv Sci (Weinh). 2023. PMID: 37386797 Free PMC article.
-
Nitrogen-Doped Ultrananocrystalline Diamond - Optoelectronic Biointerface for Wireless Neuronal Stimulation.Adv Healthc Mater. 2025 Apr;14(9):e2403901. doi: 10.1002/adhm.202403901. Epub 2025 Feb 11. Adv Healthc Mater. 2025. PMID: 39935067 Free PMC article.
-
Optoelectronic Neural Interfaces Based on Quantum Dots.ACS Appl Mater Interfaces. 2022 May 11;14(18):20468-20490. doi: 10.1021/acsami.1c25009. Epub 2022 Apr 28. ACS Appl Mater Interfaces. 2022. PMID: 35482955 Free PMC article. Review.
References
-
- Zhang M.; Tang Z.; Liu X.; Van der Spiegel J. Electronic Neural Interfaces. Nat. Electron. 2020, 3, 191–200. 10.1038/s41928-020-0390-3. - DOI
-
- Grossman N.; Bono D.; Dedic N.; Kodandaramaiah S. B.; Rudenko A.; Suk H. J.; Cassara A. M.; Neufeld E.; Kuster N.; Tsai L. H.; Pascual-Leone A.; Boyden E. S. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041. 10.1016/j.cell.2017.05.024. - DOI - PMC - PubMed
-
1029-1041
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

