What You Need to Know Before Implementing a Clinical Research Data Warehouse: Comparative Review of Integrated Data Repositories in Health Care Institutions
- PMID: 32852280
- PMCID: PMC7484778
- DOI: 10.2196/17687
What You Need to Know Before Implementing a Clinical Research Data Warehouse: Comparative Review of Integrated Data Repositories in Health Care Institutions
Abstract
Background: Integrated data repositories (IDRs), also referred to as clinical data warehouses, are platforms used for the integration of several data sources through specialized analytical tools that facilitate data processing and analysis. IDRs offer several opportunities for clinical data reuse, and the number of institutions implementing an IDR has grown steadily in the past decade.
Objective: The architectural choices of major IDRs are highly diverse and determining their differences can be overwhelming. This review aims to explore the underlying models and common features of IDRs, provide a high-level overview for those entering the field, and propose a set of guiding principles for small- to medium-sized health institutions embarking on IDR implementation.
Methods: We reviewed manuscripts published in peer-reviewed scientific literature between 2008 and 2020, and selected those that specifically describe IDR architectures. Of 255 shortlisted articles, we found 34 articles describing 29 different architectures. The different IDRs were analyzed for common features and classified according to their data processing and integration solution choices.
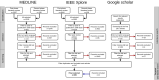
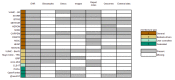
Results: Despite common trends in the selection of standard terminologies and data models, the IDRs examined showed heterogeneity in the underlying architecture design. We identified 4 common architecture models that use different approaches for data processing and integration. These different approaches were driven by a variety of features such as data sources, whether the IDR was for a single institution or a collaborative project, the intended primary data user, and purpose (research-only or including clinical or operational decision making).
Conclusions: IDR implementations are diverse and complex undertakings, which benefit from being preceded by an evaluation of requirements and definition of scope in the early planning stage. Factors such as data source diversity and intended users of the IDR influence data flow and synchronization, both of which are crucial factors in IDR architecture planning.
Keywords: data aggregation; data analytics; data warehousing; database; health informatics; information storage and retrieval.
©Kristina K Gagalova, M Angelica Leon Elizalde, Elodie Portales-Casamar, Matthias Görges. Originally published in JMIR Formative Research (http://formative.jmir.org), 27.08.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Lau F, Price M, Boyd J, Partridge C, Bell H, Raworth R. Impact of electronic medical record on physician practice in office settings: a systematic review. BMC Med Inform Decis Mak. 2012 Feb 24;12:10. doi: 10.1186/1472-6947-12-10. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-694... - DOI - PMC - PubMed
-
- MacKenzie SL, Wyatt MC, Schuff R, Tenenbaum JD, Anderson N. Practices and perspectives on building integrated data repositories: results from a 2010 CTSA survey. J Am Med Inform Assoc. 2012 Jun;19(e1):e119–24. doi: 10.1136/amiajnl-2011-000508. http://europepmc.org/abstract/MED/22437072 - DOI - PMC - PubMed
-
- Anderson N, Abend A, Mandel A, Geraghty E, Gabriel D, Wynden R, Kamerick M, Anderson K, Rainwater J, Tarczy-Hornoch P. Implementation of a deidentified federated data network for population-based cohort discovery. J Am Med Inform Assoc. 2012 Jun;19(e1):e60–7. doi: 10.1136/amiajnl-2011-000133. http://europepmc.org/abstract/MED/21873473 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

