Efficacy and Safety of 2 Fingolimod Doses vs Glatiramer Acetate for the Treatment of Patients With Relapsing-Remitting Multiple Sclerosis: A Randomized Clinical Trial
- PMID: 32852530
- PMCID: PMC7445630
- DOI: 10.1001/jamaneurol.2020.2950
Efficacy and Safety of 2 Fingolimod Doses vs Glatiramer Acetate for the Treatment of Patients With Relapsing-Remitting Multiple Sclerosis: A Randomized Clinical Trial
Abstract
Importance: Doses of fingolimod lower than 0.5 mg per day were not investigated during the fingolimod clinical development program. Whether lower doses of fingolimod might retain efficacy with fewer safety risks remains unknown.
Objective: To evaluate the efficacy and safety of fingolimod, 0.5 mg, and fingolimod, 0.25 mg, compared with glatiramer acetate and to assess whether these doses of fingolimod show superior efficacy to glatiramer acetate in adult patients with relapsing-remitting multiple sclerosis.
Interventions: Fingolimod, 0.5 mg, or fingolimod, 0.25 mg, orally once per day or glatiramer acetate, 20 mg, subcutaneously once per day.
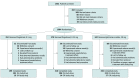
Design, setting, and participants: The Multiple Sclerosis Study Evaluating Safety and Efficacy of Two Doses of Fingolimod Versus Copaxone (ASSESS) was a phase 3b multicenter randomized rater-blinded and dose-blinded 12-month clinical trial conducted between August 9, 2012, and April 30, 2018 (including the time required to recruit participants). A total of 1461 patients aged 18 to 65 years with relapsing-remitting multiple sclerosis were screened, and 1064 participants were randomized. These participants had at least 1 documented relapse during the previous year or 2 documented relapses during the previous 2 years and an Expanded Disability Status Scale score of 0 to 6 at screening. Data were analyzed between September and November 2018.
Main outcomes and measures: The superiority of the fingolimod doses was tested hierarchically, with fingolimod, 0.5 mg, vs glatiramer acetate, 20 mg, tested first, followed by fingolimod, 0.25 mg, vs glatiramer acetate, 20 mg. The primary end point was the reduction in annualized relapse rate (ARR). Magnetic resonance imaging parameters, safety, and tolerability were also assessed.
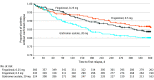
Results: Of 1461 adult patients screened, 1064 participants (72.8%) were randomized (mean [SD] age, 39.6 [11.0] years; 792 women [74.4%]) to 3 treatment groups: 352 participants received fingolimod, 0.5 mg, 370 participants received fingolimod, 0.25 mg, and 342 participants received glatiramer acetate, 20 mg. In total, 859 participants (80.7%) completed the study. Treatment with fingolimod, 0.5 mg, was superior to treatment with glatiramer acetate, 20 mg, in reducing ARR (40.7% relative reduction); the relative reduction with fingolimod, 0.25 mg, was 14.6%, which was not statistically significant (for fingolimod, 0.5 mg, ARR, 0.15; 95% CI, 0.11-0.21; for fingolimod, 0.25 mg, ARR, 0.22; 95% CI, 0.17-0.29; for glatiramer acetate, 20 mg, ARR, 0.26; 95% CI, 0.20-0.34). Treatment with both fingolimod doses (0.5 mg and 0.25 mg) significantly reduced new or newly enlarging T2 and gadolinium-enhancing T1 lesions compared with treatment with glatiramer acetate. Adverse events were reported in similar proportions across treatment groups (312 participants [90.4%] in the fingolimod, 0.5 mg, group, 323 participants [88.3%] in the fingolimod, 0.25 mg, group, and 283 participants [87.3%] in the glatiramer acetate group).
Conclusions and relevance: Fingolimod, 0.5 mg, demonstrated superior clinical efficacy compared with glatiramer acetate, 20 mg, and had a superior benefit-risk profile compared with fingolimod, 0.25 mg, in adult participants with relapsing-remitting multiple sclerosis.
Trial registration: ClinicalTrials.gov Identifier: NCT01633112.
Conflict of interest statement
Figures
References
-
- ECTRIMS 2019–late breaking news abstracts. Mult Scler J. 2019; 25(2):890-938.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

