Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial
- PMID: 32852531
- PMCID: PMC7453348
- DOI: 10.1001/jamaoncol.2020.2955
Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial
Abstract
Importance: Novel approaches are needed to improve outcomes in patients with squamous cell carcinoma of the oral cavity. Neoadjuvant immunotherapy given prior to surgery and combining programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) immune checkpoint inhibitors are 2 strategies to enhance antitumor immune responses that could be of benefit.
Design, setting, and participants: In this randomized phase 2 clinical trial conducted at 1 academic center, 29 patients with untreated squamous cell carcinoma of the oral cavity (≥T2, or clinically node positive) were enrolled between 2016 to 2019.
Interventions: Treatment was administered with nivolumab, 3 mg/kg, weeks 1 and 3, or nivolumab and ipilimumab (ipilimumab, 1 mg/kg, given week 1 only). Patients had surgery 3 to 7 days following cycle 2.
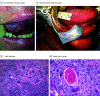
Main outcomes and measures: Safety and volumetric response determined using bidirectional measurements. Secondary end points included pathologic and objective response, progression-free survival (PFS), and overall survival. Multiplex immunofluorescence was used to evaluate primary tumor immune markers.
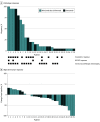
Results: Fourteen patients were randomized to nivolumab (N) and 15 patients to nivolumab/ipilimumab (N+I) (mean [SD] age, 62 [12] years; 18 men [62%] and 11 women [38%]). The most common subsite was oral tongue (n = 16). Baseline clinical staging included patients with T2 (n = 20) or greater (n = 9) T stage and 17 patients (59%) with node-positive disease. Median time from cycle 1 to surgery was 19 days (range, 7-21 days); there were no surgical delays. There were toxic effects at least possibly related to study treatment in 21 patients, including grade 3 to 4 events in 2 (N), and 5 (N+I) patients. One patient died of conditions thought unrelated to study treatment (postoperative flap failure, stroke). There was evidence of response in both the N and N+I arms (volumetric response 50%, 53%; pathologic downstaging 53%, 69%; RECIST response 13%, 38%; and pathologic response 54%, 73%, respectively). Four patients had major/complete pathologic response greater than 90% (N, n = 1; N+I, n = 3). With 14.2 months median follow-up, 1-year progression-free survival was 85% and overall survival was 89%.
Conclusions and relevance: Treatment with N and N+I was feasible prior to surgical resection. We observed promising rates of response in both arms, supporting further neoadjuvant studies with these agents.
Trial registration: ClinicalTrials.gov Identifier: NCT02919683.
Conflict of interest statement
Figures
Comment in
-
Clinical Decision-making About Neoadjuvant Nivolumab Plus Ipilimumab-Reply.JAMA Oncol. 2021 Feb 1;7(2):309-310. doi: 10.1001/jamaoncol.2020.6994. JAMA Oncol. 2021. PMID: 33355594 No abstract available.
-
Clinical Decision-making About Neoadjuvant Nivolumab Plus Ipilimumab.JAMA Oncol. 2021 Feb 1;7(2):309. doi: 10.1001/jamaoncol.2020.6989. JAMA Oncol. 2021. PMID: 33355598 No abstract available.
-
Immunotherapy in Head and Neck Cancer-Ready for Prime Time or More Research Needed?Int J Radiat Oncol Biol Phys. 2021 Mar 1;109(3):647-650. doi: 10.1016/j.ijrobp.2020.11.022. Int J Radiat Oncol Biol Phys. 2021. PMID: 33516431 Free PMC article. No abstract available.
References
-
- Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948-960. doi: 10.1016/S1470-2045(19)30151-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

