Non-invasive imaging of mouse embryo metabolism in response to induced hypoxia
- PMID: 32852649
- PMCID: PMC7468040
- DOI: 10.1007/s10815-020-01872-w
Non-invasive imaging of mouse embryo metabolism in response to induced hypoxia
Abstract
Purpose: This study used noninvasive, fluorescence lifetime imaging microscopy (FLIM)-based imaging of NADH and FAD to characterize the metabolic response of mouse embryos to short-term oxygen deprivation. We investigated the response to hypoxia at various preimplantation stages.
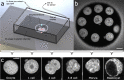
Methods: Mouse oocytes and embryos were exposed to transient hypoxia by dropping the oxygen concentration in media from 5-0% over the course of ~1.5 h, then 5% O2 was restored. During this time, FLIM-based metabolic imaging measurements of oocyte/embryo cohorts were taken every 3 minutes. Experiments were performed in triplicate for oocytes and embryos at the 1- to 8-cell, morula, and blastocyst stages. Maximum hypoxia response for each of eight measured quantitative FLIM parameters was taken from the time points immediately before oxygen restoration.
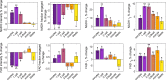
Results: Metabolic profiles showed significant changes in response to hypoxia for all stages of embryo development. The response of the eight measured FLIM parameters to hypoxia was highly stage-dependent. Of the eight FLIM parameters measured, NADH and FAD intensity showed the most dramatic metabolic responses in early developmental stages. At later stages, however, other parameters, such as NADH fraction engaged and FAD lifetimes, showed greater changes. Metabolic parameter values generally returned to baseline with the restoration of 5% oxygen.
Conclusions: Quantitative FLIM-based metabolic imaging was highly sensitive to metabolic changes induced by hypoxia. Metabolic response profiles to oxygen deprivation were distinct at different stages, reflecting differences in metabolic plasticity as preimplantation embryos develop.
Keywords: Embryo metabolism; FLIM; Hypoxia; Metabolic imaging; Mitochondria; Noninvasive assessment.
Conflict of interest statement
TS and DJN co-hold patent US20150346100A1 pending for metabolic imaging methods for assessment of oocytes and embryos and patent US20170039415A1 issued for nonlinear imaging systems and methods for assisted reproductive technologies.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

