TLR5 activation in hepatocytes alleviates the functional suppression of intrahepatic CD8+ T cells
- PMID: 32852795
- PMCID: PMC7692256
- DOI: 10.1111/imm.13251
TLR5 activation in hepatocytes alleviates the functional suppression of intrahepatic CD8+ T cells
Abstract
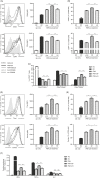
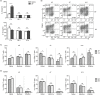
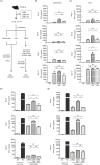
The liver is an immune-privileged organ with a tolerogenic environment for maintaining liver homeostasis. This hepatic tolerance limits the intrahepatic CD8+ T-cell response for eliminating infections. The tolerant microenvironment in the liver is orchestrated by liver-specific immunoregulatory cells that can be functionally regulated by pathogen-associated molecular patterns (PAMPs). Here, we report that flagellin, a key PAMP of gut bacteria, modulates the intrahepatic CD8+ T-cell response by activating the TLR5 signalling pathway of hepatocytes. We found that mice treated with Salmonella-derived recombinant flagellin (SF) by hydrodynamic injection had a significantly elevated IFN-γ production by the intrahepatic lymphocytes in 7 days after injection. This was correlated with a reduced immune suppressive effect of primary mouse hepatocytes (PMHs) in comparison with that of PMHs from mock-injected control mice. In vitro co-culture of SF-treated PMHs with splenocytes revealed that hepatocyte-induced immune suppression is alleviated through activation of the TLR5 but not the NLRC4 signalling pathway, leading to improved activation and function of CD8+ T cells during anti-CD3 stimulation or antigen-specific activation. In an acute HBV replication mouse model established by co-administration of SF together with an HBV-replicating plasmid by hydrodynamic injection, SF significantly enhanced the intrahepatic HBV-specific CD8+ T-cell response against HBV surface antigen. Our results clearly showed that flagellin plays a role in modulating the intrahepatic CD8+ T-cell response by activating the TLR5 pathway in PMHs, which suggests a potential role for gut bacteria in regulating liver immunity.
Keywords: CD8+ T-cell response; Toll-like receptor 5; flagellin; hepatocytes; liver tolerance.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures



References
-
- Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–18. - PubMed
-
- Spangenberg HC, Viazov S, Kersting N, Neumann‐Haefelin C, McKinney D, Roggendorf M, et al. Intrahepatic CD8+ T‐cell failure during chronic hepatitis C virus infection. Hepatology. 2005;42:828–37. - PubMed
-
- Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol. 2018;36:247–77. - PubMed
-
- Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

