Home versus inpatient induction of labour for improving birth outcomes
- PMID: 32852803
- PMCID: PMC8094591
- DOI: 10.1002/14651858.CD007372.pub4
Home versus inpatient induction of labour for improving birth outcomes
Abstract
Background: The setting in which induction of labour takes place (home or inpatient) is likely to have implications for safety, women's experiences and costs. Home induction may be started at home with the subsequent active phase of labour happening either at home or in a healthcare facility (hospital, birth centre, midwifery-led unit). More commonly, home induction starts in a healthcare facility, then the woman goes home to await the start of labour. Inpatient induction takes place in a healthcare facility where the woman stays while awaiting the start of labour.
Objectives: To assess the effects on neonatal and maternal outcomes of third trimester home induction of labour compared with inpatient induction using the same method of induction.
Search methods: For this update, we searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (31 January 2020)), and reference lists of retrieved studies.
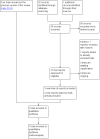
Selection criteria: Published and unpublished randomised controlled trials (RCTs) in which home and inpatient settings for induction have been compared. We included conference abstracts but excluded quasi-randomised trials and cross-over studies.
Data collection and analysis: Two review authors independently assessed study reports for inclusion. Two review authors carried out data extraction and assessment of risk of bias independently. GRADE assessments were checked by a third review author.
Main results: We included seven RCTs, six of which provided data on 1610 women and their babies. Studies were undertaken between 1998 and 2015, and all were in high- or upper-middle income countries. Most women were induced for post dates. Three studies reported government funding, one reported no funding and three did not report on their funding source. Most GRADE assessments gave very low-certainty evidence, downgrading mostly for high risk of bias and serious imprecision. 1. Home compared to inpatient induction with vaginal prostaglandin E (PGE) (two RCTs, 1028 women and babies; 1022 providing data). Although women's satisfaction may be slightly better in home settings, the evidence is very uncertain (mean difference (MD) 0.16, 95% confidence interval (CI) -0.02 to 0.34, 1 study, 399 women), very low-certainty evidence. There may be little or no difference between home and inpatient induction for other primary outcomes, with all evidence being very low certainty: - spontaneous vaginal birth (average risk ratio (RR) [aRR] 0.91, 95% CI 0.69 to 1.21, 2 studies, 1022 women, random-effects method); - uterine hyperstimulation (RR 1.19, 95% CI 0.40 to 3.50, 1 study, 821 women); - caesarean birth (RR 1.01, 95% CI 0.81 to 1.28, 2 studies, 1022 women); - neonatal infection (RR 1.29, 95% CI 0.59 to 2.82, 1 study, 821 babies); - admission to neonatal intensive care unit (NICU) (RR 1.20, 95% CI 0.50 to 2.90, 2 studies, 1022 babies). Studies did not report serious neonatal morbidity or mortality. 2. Home compared to inpatient induction with controlled release PGE (one RCT, 299 women and babies providing data). There was no information on whether the questionnaire on women's satisfaction with care used a validated instrument, but the findings presented showed no overall difference in scores. We found little or no difference between the groups for other primary outcomes, all also being very low-certainty evidence: - spontaneous vaginal birth (RR 0.94, 95% CI 0.77 to 1.14, 1 study, 299 women); - uterine hyperstimulation (RR 1.01, 95% CI 0.51 to 1.98, 1 study, 299 women); - caesarean births (RR 0.95, 95% CI 0.64 to 1.42, 1 study, 299 women); - admission to NICU (RR 1.38, 0.57 to 3.34, 1 study, 299 babies). The study did not report on neonatal infection nor serious neonatal morbidity or mortality. 3. Home compared to inpatient induction with balloon or Foley catheter (four RCTs; three studies, 289 women and babies providing data). It was again unclear whether questionnaires reporting women's experiences/satisfaction with care were validated instruments, with one study (48 women, 69% response rate) finding women were similarly satisfied. Home inductions may reduce the number of caesarean births, but the data are also compatible with a slight increase and are of very low-certainty (RR 0.64, 95% CI 0.41 to 1.01, 2 studies, 159 women). There was little or no difference between the groups for other primary outcomes with all being very low-certainty evidence: - spontaneous vaginal birth (RR 1.04, 95% CI 0.54 to 1.98, 1 study, 48 women): - uterine hyperstimulation (RR 0.45, 95% CI 0.03 to 6.79, 1 study, 48 women); - admission to NICU (RR 0.37, 95% CI 0.07 to 1.86, 2 studies, 159 babies). There were no serious neonatal infections nor serious neonatal morbidity or mortality in the one study (involving 48 babies) assessing these outcomes.
Authors' conclusions: Data on the effectiveness, safety and women's experiences of home versus inpatient induction of labour are limited and of very low-certainty. Given that serious adverse events are likely to be extremely rare, the safety data are more likely to come from very large observational cohort studies rather than relatively small RCTs.
Trial registration: ClinicalTrials.gov NCT02842879 NCT02756689 NCT02546193 NCT03934918 NCT02210598 NCT03472937 NCT03725397 NCT03806231 NCT03769610 NCT02793609 NCT03665688 NCT02975167.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Alfirevic Z: is the Co‐ordinating Editor of Cochrane Pregnancy and Childbirth and has not been involved in the editorial processing or any editorial decisions relating to this review.
Finucane E: none known.
Gyte G: I have received royalties from John Wiley & Son in respect of ‘A Cochrane Pocket Handbook – Pregnancy and Childbirth' Hofmeyr GJ et al. 2008.
Osoti A: none known.
Pileggi V: none known.
Plachcinski R: Independent PPI member of the Trial Steering Committee for the SOLVE trial: A randomised controlled trial of a Synthetic Osmotic cervical dilator for induction of Labour in comparison to dinoprostone Vaginal insErt. Funded by Medicem International, Czech Republic (
Figures









































































































Update of
-
Outpatient versus inpatient induction of labour for improving birth outcomes.Cochrane Database Syst Rev. 2013 Nov 12;(11):CD007372. doi: 10.1002/14651858.CD007372.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD007372. doi: 10.1002/14651858.CD007372.pub4. PMID: 24222365 Updated.
References
References to studies included in this review
Biem 2003 {published data only}
-
- Biem SR, Turnell RW, Olatunbosun O, Tauh M, Biem HJ. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. Journal of Obstetrics & Gynaecology Canada: JOGC 2003;25(1):23-31. - PubMed
Mohamad 2018 {published data only}
-
- Mohamad A, Ismail NA, Rahman RA, Kalok AH, Ahmad S. A comparison between in-patient and out-patient balloon catheter cervical ripening: a prospective randomised controlled trial in PPUKM. Medical Journal of Malaysia 2018;73:22.
Policiano 2017 {published data only}
-
- Policiano C, NCT02842879. Outpatient versus inpatient cervix priming with Foley catheter. https://clinicaltrials.gov/ct2/show/NCT02842879 (first received 25 July 2016). - PubMed
-
- Policiano C, Pimenta M, Martins D, Clode N. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2017;210:1-6. - PubMed
Ryan 1998 {published data only}
-
- Ryan G, Oskamp M, Seaward PG, Barrett J, Barrett H, O'Brien K, et al. Randomized controlled trial of inpatient vs. outpatient administration of prostaglandin E2, gel for induction of labour at term [SPO Abstract 303]. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S92.
Sciscione 2001 {published data only}
-
- Pollock M, Maas B, Muench M, Sciscione A. Patient acceptance of outpatient pre-induction cervical ripening with the foley bulb. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S136.
-
- Sciscione AC, Muench M, Pollock M, Jenkins TM, Tildon-Burton J, Colmorgen GH. Transcervical foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstetrics & Gynecology 2001;98:751-6. - PubMed
Wilkinson 2015a ‐ COPRA {published data only}
-
- Adelson P, Wilkinson C, Turnbull D. Pilot trial of balloon catheter cervical priming: Women and clinician views of inpatient and outpatient applications. Journal of Paediatrics and Child Health 2014;50(Suppl 1):62-3.
-
- Turnbull D, ACTRN12612001184864. A pilot randomised controlled trial of outpatient balloon catheter priming for induction of labour. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=1261... (first received 30 October 2012).
-
- Wilkinson C, Adelson P, Turnbull D. A pilot randomised controlled trial of outpatient compared to inpatient cervical priming using double balloon catheters-clinical results of the copra study. Journal of Paediatrics and Child Health 2014;50(Suppl 1):37, Abstract no: A241.
-
- Wilkinson C, Adelson P, Turnbull D. Outpatient compared to inpatient cervical ripening with a double balloon catheter. A pilot randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S1):231. - PubMed
Wilkinson 2015 ‐ OPRA {published data only}
-
- Adelson L, Wedlock R, Wilkinson S, Howard K, Bryce L, Turnbull A. A cost analysis of inpatient compared with outpatient prostaglandin E2 cervical priming for induction of labour: results from the OPRA trial. Australian Health Review 2013;37(4):467-73. - PubMed
-
- Adelson P, Wedlock G, Wilkinson C, Bryce R, Turnbull D. A cost analysis of outpatient priming for induction of labour induction: Results from the outpatient priming for induction of labour trial (OPRA). Journal of Paediatrics and Child Health 2013;49 Suppl 2:27. - PubMed
-
- Turnbull D, Adelson P, Oster C, Bryce R, Fereday J, Wilkinson C. Psychosocial outcomes of a randomized controlled trial of outpatient cervical priming for induction of labor. Birth 2013;40(2):75-80. - PubMed
-
- Turnbull D, Adelson P, Stamp G, Fereday J, Ryan P, Bryce R, et al. A two-centre randomised controlled trial of outpatient cervical priming for induction of labour: Psychosocial outcomes. Journal of Paediatrics and Child Health 2012;48(Suppl 1):61.
References to studies excluded from this review
Austin 2015 {published data only}
-
- Austin K, Chambers GM, De Abreu Lourenco R, Madan A, Susic D, Henry A. Cost-effectiveness of term induction of labour using inpatient prostaglandin gel versus outpatient Foley catheter. Australian & New Zealand Journal of Obstetrics & Gynaecology 2015;55(5):440-5. - PubMed
Beckmann 2020 {published data only}
-
- Beckmann M, ACTRN12614000039684. Prostaglandin Inpatient iNduction of labour Compared with BALLOon Outpatient iNduction of labour: a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=1261... (first received 19 December 2013).
-
- Beckmann M, Acreman M, Schmidt E, Merollini KM, Miller Y. Women's experience of induction of labor using PGE2 as an inpatient versus balloon catheter as an outpatient. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2020;249:1-6. [CENTRAL: CN-02118449] [PMID: ] - PubMed
-
- Beckmann M, Gibbons K, Flenady V, Kumar S. Induction of labour using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicentre randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2020;127(5):571-9. [CENTRAL: CN-02073515] [EMBASE: 630016333] [PMID: ] - PubMed
Henry 2011 {published data only}
-
- Henry A, ACTRN12609000420246. An evaluation of Outpatient Foley (intracervical) catheter versus Inpatient Prostaglandin Vaginal Gel (PGE2) on the induction of labour at term. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000... (first received 10 May 2009).
-
- Henry A, Madan A, Reid R, Tracy S, Sharpe V, Austin K, et al. Outpatient Foley catheter versus inpatient Prostin gel for cervical ripening: the FOG (Foley or Gel) trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51:473-4.
-
- Henry A, Reid R, Madan A, Tracy S, Sharpe V, Welsh A, et al. Satisfaction survey: outpatient Foley catheter versus inpatient Prostin gel for cervical ripening. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51:474.
Kuper 2018 {published data only}
-
- Kuper SG, Jauk VC, George DM, Edwards RK, Szychowski JM, Mazzoni SE, et al. Outpatient Foley catheter for induction of labor in parous women a randomized controlled trial. Obstetrics and Gynecology 2018;132(1):94-101. - PubMed
-
- Kuper SG, NCT02756689. Outpatient Foley for starting induction of labor at term. https://clinicaltrials.gov/ct2/show/NCT02756689 (first received 29 April 2016).
-
- Subramaniam A, Blanchard CT, Kuper SG, Jauk VC, Szychowski JM, Tita AT, et al. 660: Outpatient versus inpatient cervical ripening in obese parous women. American Journal of Obstetrics and Gynecology 2019;220(1):S437.
PonMalar 2017 {published data only}
-
- PonMalar J, Benjamin SJ, Abraham A, Rathore S, Jeyaseelan V, Mathews JE. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 microg of misoprostol in the outpatient setting to prevent formal induction of labour. Archives of Gynecology and Obstetrics 2017;295(1):33-8. - PubMed
Rijnders 2011 {published data only}ISRCTN47736435
-
- Rijnders ME, ISRCTN47736435. Costs and effects of amniotomy at home for induction of post term pregnancy. http://www.isrctn.com/ISRCTN47736435 (9 January 2006).
-
- Rijnders ME. A randomised controlled trial of amniotomy at home for induction between 292 and 294 days gestation. In: Interventions in Midwife Led Care in the Netherlands to Achieve Optimal Birth Outcomes: Effects and Women's Experiences. Amsterdam: University of Amsterdam, 2011.
Torbenson 2015 {published data only}
-
- Torbenson VE, NCT02546193. Outpatient Foley catheter compared to usual inpatient care for cervical ripening: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT02546193 (first received 10 September 2015).
Wise 2020 {published data only}
-
- Wise M, ACTRN12616000739415. Comparison of low-risk pregnant women undergoing induction of labour at term by outpatient balloon or inpatient prostaglandin in order to assess caesarean section rate; a randomised controlled trial. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000... (first received 15 March 2016).
References to studies awaiting assessment
Denona 2018 TR {published data only}ISRCTN10109823
-
- Denona B, ISRCTN10109823. Comparison on patient satisfaction with Cervical Ripening Balloon using inpatient and outpatient protocol. http://www.isrctn.com/ISRCTN10109823 (first received 24 April 2018).
Hedriana 2019 TR {published data only}
-
- Hedriana H, NCT03934918. Outpatient cervical preparation to reduce induction duration in nulliparous term singleton vertex women: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03934918 (first received 2 May 2019).
Mullin 2014 TR {published data only}
-
- Mullin PM, NCT02210598. Outpatient labor induction with the transcervical Foley balloon: a randomized trial comparing outpatient immediate removal Foley versus standard inpatient Foley induction. https://clinicaltrials.gov/ct2/show/NCT02210598 (first received 6 August 2014).
References to ongoing studies
Ausbeck 2018 {published data only}
-
- Ausbeck EB, NCT03472937. Outpatient Foley for starting induction of labor at term in nulliparous women: a randomized-controlled study. https://clinicaltrials.gov/ct2/show/NCT03472937 (first received 21 March 2018).
Kohari 2018 {published data only}
-
- Kohari K, NCT03725397. Inpatient versus outpatient Foley cervical ripening study. https://clinicaltrials.gov/ct2/show/NCT03725397 (first received 31 October 2018).
Mishra 2007 {published data only}ISRCTN71488751
-
- ISRCTN71488751. A prospective randomised controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. http://www.isrctn.com/ISRCTN71488751 (first received 2007). - PubMed
Nichols 2019 {published data only}
-
- Nichols J, NCT03806231. A trial of Cervidil (dinoprostone, prostaglandin E2 (PGE2), insert) for outpatient pre-induction of cervical ripening in women at 39.0-41.6 weeks gestation. https://clinicaltrials.gov/ct2/show/NCT03806231 (first received 16 January 2019).
Pierce‐Williams 2018 {published data only}
-
- Pierce-Williams R, NCT03769610. Inpatient versus outpatient transcervical Foley catheter use for cervical ripening: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03769610 (first received 7 December 2018).
Pin 2018 {published data only}ISRCTN13534944
-
- Pin TY, ISRCTN13534944. Induction of labour with a foley catheter in pregnant women with previous successful vaginal birth in outpatient vs inpatient settings. http://www.isrctn.com/ISRCTN13534944 (first received 18 December 2018).
Rinne 2016 {published data only}
-
- Rinne KM, NCT02793609. Outpatient versus inpatient double balloon catheter for induction of labor: a randomised trial. https://clinicaltrials.gov/ct2/show/NCT02793609 (first received 8 June 2016).
Saad 2018 {published data only}
-
- Saad A, NCT03665688. Induction of labor in women with unfavorable cervix: randomized controlled trial comparing outpatient to inpatient cervical ripening using Dilapan-S® (HOMECARE). https://clinicaltrials.gov/ct2/show/NCT03665688 (first received 11 September 2018).
Shrivastava 2016 {published data only}
-
- Shrivastava V, NCT02975167. Patient satisfaction during outpatient versus inpatient Foley catheter induction of labor. https://clinicaltrials.gov/ct2/show/NCT02975167 (first received 29 November 2016).
Additional references
ACOG 2009
-
- American College of Obstetricians and Gynecologists. Induction of Labor. ACOG Practice Bulletin 2009;114:386-97.
Alfirevic 2016
Calder 1998
-
- Calder AA. Nitric oxide--another factor in cervical ripening. Human Reproduction 1998;13(2):250-1. - PubMed
Coates 2019
-
- Coates R, Cupples G, Scamell A, McCourt C. Women’s experiences of induction of labour: qualitative systematic review and thematic synthesis. Midwifery 2019;69:17-28. - PubMed
Curtis 1987
-
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91-5. - PubMed
Dong 2020
Dos Santos 2018
-
- Dos Santos F, Drymiotou S, Antequera Martin A, Mol BW, Gale C, Devane D, et al. Development of a core outcome set for trials on induction of labour: an international multistakeholder Delphi study. British Journal of Obstetrics and Gynaecology 2018;125:1673–80. - PubMed
Finucane 2020
Gates 2005
-
- Gates S. Methodological Guidelines. In: The Editorial Team. Pregnancy and Childbirth Group. About The Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2005, Issue 2.
Grobman 2018
-
- Grobman, W, Rice M, Reddy U, Tita A, Silver R, Mallett G, et al. A randomized trial of elective induction of labor at 39 weeks compared with expectant management of low-risk nulliparous women. American Journal of Obstetrics and Gynecology 2018;218(1):S601. [DOI: 10.1016/j.ajog.2017.12.016] - DOI
Higgins 2011
-
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hofmeyr 2009
-
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD002074. [DOI: 10.1002/14651858.CD002074.pub2] - DOI
Liu 2019
Lyrenas 2001
-
- Lyrenas S, Clason I, Ulmsten U. In vivo controlled release of PGE2 from a vaginal insert (0.8 mm, 10 mg) during induction of labour. British Journal of Obstetrics and Gynaecology 2001;108:169-78. - PubMed
Middleton 2018
NICE 2008
-
- National Institute of Health and Care Excellence. Inducting labour: Clinical Guideline. National Collaborating Centre for Women’s and Children’s Health 2008;RCOG Press, London:Available at https://www.nice.org.uk/guidance/cg70/chapter/4-research-recommendations.
Nilvér 2017
Nippita 2015
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Santesso 2020
-
- Santesso N, Glenton C, Dahm P, Gardner P, Akl E, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014. Epub 2019 Nov 9.PMID: 31711912] - PubMed
SOGC 2013
-
- Society of Obstetricians and Gynaecologists of Canada. Induction of Labour SOGC Clinical Practice Guideline. Society of Obstetricians and Gynaecologists of Canada 2013;296:Available at: https://sogc.org/wp-content/uploads/2013/08/September2013-CPG296-ENG-Onl... (accessed 4th April 2017).
Sue‐A‐Quan 1999
Thomas 2014
Turnbull 1996
-
- Turnbull D, Holmes A, Shields N, Cheyne H, Twaddle S, Gilmour WH, et al. Randomised, controlled trial of efficacy of midwife-managed care. Lancet 1996;348(9022):213-8. - PubMed
Vogel 2017
WHO 2011
-
- World Health Organization. WHO Recommendations for Induction of Labour. World Health Organization Guidelines 2011:http://apps.who.int/iris/bitstream/10665/44531/1/9789241501156_eng.pdf (accessed 4th April 2017).
Wong 2002
-
- Wong SF, Hui SK, Choi H, Ho LC. Does sweeping of membranes beyond 40 weeks reduce the need for formal induction of labour? British Journal of Obstetrics and Gynaecology 2002;109:632-6. - PubMed
References to other published versions of this review
Kelly 2008
Kelly 2009b
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

