Adrenal artery ablation for primary aldosteronism without apparent aldosteronoma: An efficacy and safety, proof-of-principle trial
- PMID: 32852871
- PMCID: PMC8029691
- DOI: 10.1111/jch.13960
Adrenal artery ablation for primary aldosteronism without apparent aldosteronoma: An efficacy and safety, proof-of-principle trial
Abstract
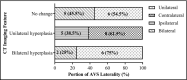
Primary aldosteronism (PA) is associated with resistant hypertension and cardiovascular events. There are some limitations of current medical and surgical therapies for PA. To determine the efficacy and safety of catheter-based adrenal artery ablation for treatment of PA patients who refused both surgery and medical therapy, we performed this prospective cohort study. Thirty-six PA patients without apparent aldosteronoma were treated by adrenal artery ablation. Primary outcome was postoperative blood pressure and defined daily dose (DDD) of antihypertensive medications after adrenal ablation. Secondary outcome was biochemical success. We assessed outcomes based on Primary Aldosteronism Surgical Outcome (PASO) criteria. Adrenal CT scan, biochemical evaluation, adrenal artery ablation and adrenal venous sampling (AVS) were underwent. After adrenal ablation, complete clinical success (normotension without antihypertensive medication) was achieved in 9/36 (25.0%) patients and partial clinical success (reduction in blood pressure or less antihypertensive medication) in 13/36 (36.1%) patients. Complete biochemical success (correction of hypokalemia and normalization of aldosterone-to-renin ratio) was achieved in 16/36 (44.4%) patients. Office-based and ambulatory blood pressures were reduced by 17/7 and 11/2 mmHg at 6 months after ablation, respectively. The plasma cortisol level in the ablation group decreased slightly, but no patient developed hypoadrenocorticism. Catheter-based adrenal ablation appears to produce substantial and sustained blood pressure reduction and biochemical improvement, with only minor adverse events in PA patients without apparent aldosteronoma. This therapy could be an important supplement for current PA treatments.
Keywords: adrenal artery ablation; antihypertensive therapy; efficacy and safety; primary aldosteronism.
©2020 Wiley Periodicals LLC.
Conflict of interest statement
None.
Figures
References
-
- Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889‐1916. - PubMed
-
- Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285(2):126‐148. - PubMed
-
- Rossi GP. Primary aldosteronism: JACC State‐of‐the‐Art Review. J Am Coll Cardiol. 2019;74(22):2799‐2811. - PubMed
-
- Mulatero P, Monticone S, Bertello C, et al. Long‐term cardio‐ and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826‐4833. - PubMed
-
- Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2018;6(1):41‐50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

