Tendon-derived cathepsin K-expressing progenitor cells activate Hedgehog signaling to drive heterotopic ossification
- PMID: 32853181
- PMCID: PMC7685727
- DOI: 10.1172/JCI132518
Tendon-derived cathepsin K-expressing progenitor cells activate Hedgehog signaling to drive heterotopic ossification
Abstract
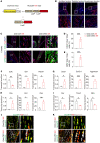
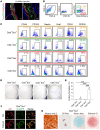
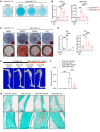
Heterotopic ossification (HO) is pathological bone formation characterized by ossification within muscle, tendons, or other soft tissues. However, the cells of origin and mechanisms involved in the pathogenesis of HO remain elusive. Here we show that deletion of suppressor of fused (Sufu) in cathepsin K-Cre-expressing (Ctsk-Cre-expressing) cells resulted in spontaneous and progressive ligament, tendon, and periarticular ossification. Lineage tracing studies and cell functional analysis demonstrated that Ctsk-Cre could label a subpopulation of tendon-derived progenitor cells (TDPCs) marked by the tendon marker Scleraxis (Scx). Ctsk+Scx+ TDPCs are enriched for tendon stem cell markers and show the highest self-renewal capacity and differentiation potential. Sufu deficiency caused enhanced chondrogenic and osteogenic differentiation of Ctsk-Cre-expressing tendon-derived cells via upregulation of Hedgehog (Hh) signaling. Furthermore, pharmacological intervention in Hh signaling using JQ1 suppressed the development of HO. Thus, our results show that Ctsk-Cre labels a subpopulation of TDPCs contributing to HO and that their cell-fate changes are driven by activation of Hh signaling.
Keywords: Bone disease; Mouse models; Stem cells; Therapeutics.
Conflict of interest statement
Figures



References
-
- Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

