The role of patiromer: Comparing OPAL-HK data with untreated real-world patients in the United Kingdom-A retrospective, propensity-matched analysis
- PMID: 32853209
- PMCID: PMC7451519
- DOI: 10.1371/journal.pone.0237467
The role of patiromer: Comparing OPAL-HK data with untreated real-world patients in the United Kingdom-A retrospective, propensity-matched analysis
Abstract
Objectives: The first phase of the published OPAL-HK study was a single-group treatment phase, which showed that patiromer normalised serum potassium at 4weeks in patients with chronic kidney disease stages 3-4 who were receiving renin-angiotensin-aldosterone inhibitors. We utilised real-world data to provide a control comparison to evaluate patiromer's efficacy in lowering serum potassium.
Materials and methods: The Salford Kidney Study (SKS) in the United Kingdom provided a matched cohort. After applying OPAL-HK inclusion and exclusion criteria, patients with an outpatient potassium level between 5.1mmol/L to <6.5mmol/L and whose next outpatient level was checked 24-42 days later were selected. Patients underwent 1:1 matching with the 243 OPAL-HK patients using propensity matching based on 6 variables: age, gender, estimated glomerular filtration rate, diabetes, heart failure and potassium level. The study outcomes aligned with the OPAL-HK treatment phase: mean change in baseline potassium, and the proportion of patients with a potassium of 3.8 to <5.1mmol/L at follow-up.
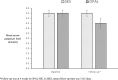
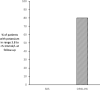
Results: The study comprised 87 precisely matched patients. The mean follow-up in the 87 SKS patients was 31±5 days. At baseline, matched patients had a mean potassium of 5.5±0.3mmol/L. At follow-up, the mean level was unchanged in SKS patients but was 4.5±0.5mmol/L in the OPAL-HK group (p<0.001), a mean (±SE) change of -1.00±0.06mmol/L. The target range of 3.8 to <5.1mmol/L was reached in 80% of OPAL-HK patients compared with 0% in the SKS cohort. There were very few interventions undertaken to reduce hyperkalaemia in SKS patients.
Conclusions: Using real-world data as a matched control arm for the first phase of the OPAL-HK study, we highlight a potential role for patiromer in lowering potassium levels in patients with CKD 3-4 receiving renin-angiotensin-aldosterone inhibitors.
Conflict of interest statement
PK received research grants and speaker/consultancy fees from Vifor Fresenius Medical Care Renal Pharma outside the submitted work. GC is an employee and holds shares of the Vifor Pharma Group. MI was an employee of the Vifor Pharma Group from September 2018 to May 2020. Vifor Pharma markets patiromer. These declarations do not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors.Eur J Heart Fail. 2015 Oct;17(10):1057-65. doi: 10.1002/ejhf.402. Epub 2015 Oct 12. Eur J Heart Fail. 2015. PMID: 26459796 Free PMC article.
-
Treatment with patiromer decreases aldosterone in patients with chronic kidney disease and hyperkalemia on renin-angiotensin system inhibitors.Kidney Int. 2016 Sep;90(3):696-704. doi: 10.1016/j.kint.2016.04.019. Epub 2016 Jun 24. Kidney Int. 2016. PMID: 27350174 Clinical Trial.
-
Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors.N Engl J Med. 2015 Jan 15;372(3):211-21. doi: 10.1056/NEJMoa1410853. Epub 2014 Nov 21. N Engl J Med. 2015. PMID: 25415805 Clinical Trial.
-
Patiromer: A Review in Hyperkalaemia.Clin Drug Investig. 2016 Aug;36(8):687-94. doi: 10.1007/s40261-016-0432-9. Clin Drug Investig. 2016. PMID: 27380495 Review.
-
Patiromer: a clinical review.Curr Med Res Opin. 2016;32(1):155-64. doi: 10.1185/03007995.2015.1106935. Epub 2015 Nov 19. Curr Med Res Opin. 2016. PMID: 26456884 Review.
Cited by
-
Proteomic signature associated with chronic kidney disease (CKD) progression identified by data-independent acquisition mass spectrometry.Clin Proteomics. 2023 Apr 20;20(1):19. doi: 10.1186/s12014-023-09405-0. Clin Proteomics. 2023. PMID: 37076799 Free PMC article.
-
Renin-angiotensin-aldosterone pathway modulators in chronic kidney disease: A comparative review.Front Pharmacol. 2023 Feb 13;14:1101068. doi: 10.3389/fphar.2023.1101068. eCollection 2023. Front Pharmacol. 2023. PMID: 36860293 Free PMC article. Review.
References
-
- KDIGO: Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int 2013;3(1):i–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical