Development and evaluation of a rapid CRISPR-based diagnostic for COVID-19
- PMID: 32853291
- PMCID: PMC7451577
- DOI: 10.1371/journal.ppat.1008705
Development and evaluation of a rapid CRISPR-based diagnostic for COVID-19
Abstract
The recent outbreak of human infections caused by SARS-CoV-2, the third zoonotic coronavirus has raised great public health concern globally. Rapid and accurate diagnosis of this novel pathogen posts great challenges not only clinically but also technologically. Metagenomic next-generation sequencing (mNGS) and reverse-transcription PCR (RT-PCR) have been the most commonly used molecular methodologies. However, each has their own limitations. In this study, we developed an isothermal, CRISPR-based diagnostic for COVID-19 with near single-copy sensitivity. The diagnostic performances of all three technology platforms were also compared. Our study aimed to provide more insights into the molecular detection of SARS-CoV-2, and also to present a novel diagnostic option for this new emerging virus.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
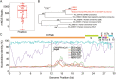


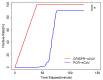
Similar articles
-
iSCAN: An RT-LAMP-coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-2.Virus Res. 2020 Oct 15;288:198129. doi: 10.1016/j.virusres.2020.198129. Epub 2020 Aug 18. Virus Res. 2020. PMID: 32822689 Free PMC article.
-
Detecting SARS-CoV-2 at point of care: preliminary data comparing loop-mediated isothermal amplification (LAMP) to polymerase chain reaction (PCR).BMC Infect Dis. 2020 Oct 20;20(1):783. doi: 10.1186/s12879-020-05484-8. BMC Infect Dis. 2020. PMID: 33081710 Free PMC article.
-
Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay.Clin Microbiol Infect. 2020 Jun;26(6):773-779. doi: 10.1016/j.cmi.2020.04.001. Epub 2020 Apr 8. Clin Microbiol Infect. 2020. PMID: 32276116 Free PMC article.
-
Current and Perspective Diagnostic Techniques for COVID-19.ACS Infect Dis. 2020 Aug 14;6(8):1998-2016. doi: 10.1021/acsinfecdis.0c00365. Epub 2020 Aug 4. ACS Infect Dis. 2020. PMID: 32677821 Review.
-
Recent advances in the nucleic acid-based diagnostic tool for coronavirus.Mol Biol Rep. 2020 Nov;47(11):9033-9041. doi: 10.1007/s11033-020-05889-3. Epub 2020 Oct 6. Mol Biol Rep. 2020. PMID: 33025503 Free PMC article. Review.
Cited by
-
Mass molecular testing for COVID19 using NGS-based technology and a highly scalable workflow.Sci Rep. 2021 Mar 29;11(1):7122. doi: 10.1038/s41598-021-86498-3. Sci Rep. 2021. PMID: 33782491 Free PMC article.
-
An engineered CRISPR-Cas12a variant and DNA-RNA hybrid guides enable robust and rapid COVID-19 testing.Nat Commun. 2021 Mar 19;12(1):1739. doi: 10.1038/s41467-021-21996-6. Nat Commun. 2021. PMID: 33741959 Free PMC article.
-
Loop-Mediated Isothermal Amplification-Integrated CRISPR Methods for Infectious Disease Diagnosis at Point of Care.ACS Omega. 2023 Nov 7;8(46):43357-43373. doi: 10.1021/acsomega.3c04422. eCollection 2023 Nov 21. ACS Omega. 2023. PMID: 38027359 Free PMC article. Review.
-
Molecular and Serologic Diagnostic Technologies for SARS-CoV-2.ArXiv [Preprint]. 2022 Apr 26:arXiv:2204.12598v2. ArXiv. 2022. PMID: 35547240 Free PMC article. Preprint.
-
CRISPR-cas13 enzymology rapidly detects SARS-CoV-2 fragments in a clinical setting.J Clin Virol. 2021 Dec;145:105019. doi: 10.1016/j.jcv.2021.105019. Epub 2021 Oct 28. J Clin Virol. 2021. PMID: 34753073 Free PMC article.
References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

