Mechanism of ventricular premature beats elicited by left stellate ganglion stimulation during acute ischaemia of the anterior left ventricle
- PMID: 32853334
- PMCID: PMC8318107
- DOI: 10.1093/cvr/cvaa253
Mechanism of ventricular premature beats elicited by left stellate ganglion stimulation during acute ischaemia of the anterior left ventricle
Abstract
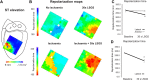
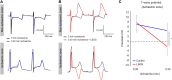
Aims: Enhanced sympathetic activity during acute ischaemia is arrhythmogenic, but the underlying mechanism is unknown. During ischaemia, a diastolic current flows from the ischaemic to the non-ischaemic myocardium. This 'injury' current can cause ventricular premature beats (VPBs) originating in the non-ischaemic myocardium, especially during a deeply negative T wave in the ischaemic zone. We reasoned that shortening of repolarization in myocardium adjacent to ischaemic myocardium increases the 'injury' current and causes earlier deeply negative T waves in the ischaemic zone, and re-excitation of the normal myocardium. We tested this hypothesis by activation and repolarization mapping during stimulation of the left stellate ganglion (LSG) during left anterior descending coronary artery (LAD) occlusion.
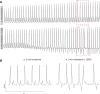
Methods and results: In nine pigs, five subsequent episodes of acute ischaemia, separated by 20 min of reperfusion, were produced by occlusion of the LAD and 121 epicardial local unipolar electrograms were recorded. During the third occlusion, left stellate ganglion stimulation (LSGS) was initiated after 3 min for a 30-s period, causing a shortening of repolarization in the normal myocardium by about 100 ms. This resulted in more negative T waves in the ischaemic zone and more VPBs than during the second, control, occlusion. Following the decentralization of the LSG (including removal of the right stellate ganglion and bilateral cervical vagotomy), fewer VPBs occurred during ischaemia without LSGS. During LSGS, the number of VPBs was similar to that recorded before decentralization.
Conclusion: LSGS, by virtue of shortening of repolarization in the non-ischaemic myocardium by about 100 ms, causes deeply negative T waves in the ischaemic tissue and VPBs originating from the normal tissue adjacent to the ischaemic border.
Keywords: Arrhythmias; Autonomic nervous system; Injury current; Ischaemia; Repolarization.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures





References
-
- Verrier RL, Lown B.. Influence of neural activity on ventricular electrical stability during acute myocardial ischemia and infarction. In Sandoe E., Julian D.G., Bell J.W. (eds) Management of Ventricular Tachycardia—Role of Mexilitine. Amsterdam: Excerpta Medica, 1978. pp. 133–150.
-
- Corr PB, Yamada KA, Witkowski FX.. Mechanisms controlling cardiac autonomic function and their relation to arrhythmogenesis. In Fozzard F.A., Haber E., Jennings R.B., Katz A.M., Morgan H.E. (eds) The Heart and Cardiovascular System. New York: Raven Press, 1986. pp. 1343–1404.
-
- Schwartz PJ. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat Rev Cardiol 2014;11:346–353. - PubMed

