Ultra-High-Dose-Rate FLASH Irradiation Limits Reactive Gliosis in the Brain
- PMID: 32853387
- PMCID: PMC7856066
- DOI: 10.1667/RADE-20-00067.1
Ultra-High-Dose-Rate FLASH Irradiation Limits Reactive Gliosis in the Brain
Abstract
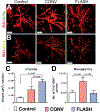
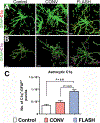
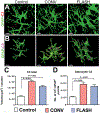
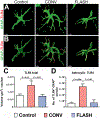
Encephalic radiation therapy delivered at a conventional dose rate (CONV, 0.1-2.0 Gy/min) elicits a variety of temporally distinct damage signatures that invariably involve persistent indications of neuroinflammation. Past work has shown an involvement of both the innate and adaptive immune systems in modulating the central nervous system (CNS) radiation injury response, where elevations in astrogliosis, microgliosis and cytokine signaling define a complex pattern of normal tissue toxicities that never completely resolve. These side effects constitute a major limitation in the management of CNS malignancies in both adult and pediatric patients. The advent of a novel ultra-high dose-rate irradiation modality termed FLASH radiotherapy (FLASH-RT, instantaneous dose rates ≥106 Gy/s; 10 Gy delivered in 1-10 pulses of 1.8 µs) has been reported to minimize a range of normal tissue toxicities typically concurrent with CONV exposures, an effect that has been coined the "FLASH effect." Since the FLASH effect has now been found to significantly limit persistent inflammatory signatures in the brain, we sought to further elucidate whether changes in astrogliosis might account for the differential dose-rate response of the irradiated brain. Here we report that markers selected for activated astrogliosis and immune signaling in the brain (glial fibrillary acidic protein, GFAP; toll-like receptor 4, TLR4) are expressed at reduced levels after FLASH irradiation compared to CONV-irradiated animals. Interestingly, while FLASH-RT did not induce astrogliosis and TLR4, the expression level of complement C1q and C3 were found to be elevated in both FLASH and CONV irradiation modalities compared to the control. Although functional outcomes in the CNS remain to be cross-validated in response to the specific changes in protein expression reported, the data provide compelling evidence that distinguishes the dose-rate response of normal tissue injury in the irradiated brain.
©2020 by Radiation Research Society. All rights of reproduction in any form reserved.
Figures

References
-
- Harrington KJ. Ultrahigh dose-rate radiotherapy: Next steps for FLASH-RT. Clin Cancer Res 2019; 25:3–5. - PubMed
-
- Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol 2018; 129:582–8. - PubMed
-
- Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother Oncol 2017; 124:365–9. - PubMed

