Interventions for American cutaneous and mucocutaneous leishmaniasis
- PMID: 32853410
- PMCID: PMC8094931
- DOI: 10.1002/14651858.CD004834.pub3
Interventions for American cutaneous and mucocutaneous leishmaniasis
Abstract
Background: On the American continent, cutaneous and mucocutaneous leishmaniasis (CL and MCL) are diseases associated with infection by several species of Leishmania parasites. Pentavalent antimonials remain the first-choice treatment. There are alternative interventions, but reviewing their effectiveness and safety is important as availability is limited. This is an update of a Cochrane Review first published in 2009.
Objectives: To assess the effects of interventions for all immuno-competent people who have American cutaneous and mucocutaneous leishmaniasis (ACML).
Search methods: We updated our database searches of the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, LILACS and CINAHL to August 2019. We searched five trials registers.
Selection criteria: Randomised controlled trials (RCTs) assessing either single or combination treatments for ACML in immuno-competent people, diagnosed by clinical presentation and Leishmania infection confirmed by smear, culture, histology, or polymerase chain reaction on a biopsy specimen. The comparators were either no treatment, placebo only, or another active compound.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Our key outcomes were the percentage of participants 'cured' at least three months after the end of treatment, adverse effects, and recurrence. We used GRADE to assess evidence certainty for each outcome.
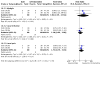
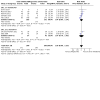
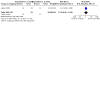
Main results: We included 75 studies (37 were new), totalling 6533 randomised participants with ATL. The studies were mainly conducted in Central and South America at regional hospitals, local healthcare clinics, and research centres. More male participants were included (mean age: roughly 28.9 years (SD: 7.0)). The most common confirmed species were L. braziliensis, L. panamensis, and L. mexicana. The most assessed interventions and comparators were non-antimonial systemics (particularly oral miltefosine) and antimonials (particularly meglumine antimoniate (MA), which was also a common intervention), respectively. Three studies included moderate-to-severe cases of mucosal leishmaniasis but none included cases with diffuse cutaneous or disseminated CL, considered the severe cutaneous form. Lesions were mainly ulcerative and located in the extremities and limbs. The follow-up (FU) period ranged from 28 days to 7 years. All studies had high or unclear risk of bias in at least one domain (especially performance bias). None of the studies reported the degree of functional or aesthetic impairment, scarring, or quality of life. Compared to placebo, at one-year FU, intramuscular (IM) MA given for 20 days to treat L. braziliensis and L. panamensis infections in ACML may increase the likelihood of complete cure (risk ratio (RR) 4.23, 95% confidence interval (CI) 0.84 to 21.38; 2 RCTs, 157 participants; moderate-certainty evidence), but may also make little to no difference, since the 95% CI includes the possibility of both increased and reduced healing (cure rates), and IMMA probably increases severe adverse effects such as myalgias and arthralgias (RR 1.51, 95% CI 1.17 to 1.96; 1 RCT, 134 participants; moderate-certainty evidence). IMMA may make little to no difference to the recurrence risk, but the 95% CI includes the possibility of both increased and reduced risk (RR 1.79, 95% CI 0.17 to 19.26; 1 RCT, 127 participants; low-certainty evidence). Compared to placebo, at six-month FU, oral miltefosine given for 28 days to treat L. mexicana, L. panamensis and L. braziliensis infections in American cutaneous leishmaniasis (ACL) probably improves the likelihood of complete cure (RR 2.25, 95% CI 1.42 to 3.38), and probably increases nausea rates (RR 3.96, 95% CI 1.49 to 10.48) and vomiting (RR 6.92, 95% CI 2.68 to 17.86) (moderate-certainty evidence). Oral miltefosine may make little to no difference to the recurrence risk (RR 2.97, 95% CI 0.37 to 23.89; low-certainty evidence), but the 95% CI includes the possibility of both increased and reduced risk (all based on 1 RCT, 133 participants). Compared to IMMA, at 6 to 12 months FU, oral miltefosine given for 28 days to treat L. braziliensis, L. panamensis, L. guyanensis and L. amazonensis infections in ACML may make little to no difference to the likelihood of complete cure (RR 1.05, 95% CI 0.90 to 1.23; 7 RCTs, 676 participants; low-certainty evidence). Based on moderate-certainty evidence (3 RCTs, 464 participants), miltefosine probably increases nausea rates (RR 2.45, 95% CI 1.72 to 3.49) and vomiting (RR 4.76, 95% CI 1.82 to 12.46) compared to IMMA. Recurrence risk was not reported. For the rest of the key comparisons, recurrence risk was not reported, and risk of adverse events could not be estimated. Compared to IMMA, at 6 to 12 months FU, oral azithromycin given for 20 to 28 days to treat L. braziliensis infections in ACML probably reduces the likelihood of complete cure (RR 0.51, 95% CI 0.34 to 0.76; 2 RCTs, 93 participants; moderate-certainty evidence). Compared to intravenous MA (IVMA) and placebo, at 12 month FU, adding topical imiquimod to IVMA, given for 20 days to treat L. braziliensis, L. guyanensis and L. peruviana infections in ACL probably makes little to no difference to the likelihood of complete cure (RR 1.30, 95% CI 0.95 to 1.80; 1 RCT, 80 participants; moderate-certainty evidence). Compared to MA, at 6 months FU, one session of local thermotherapy to treat L. panamensis and L. braziliensis infections in ACL reduces the likelihood of complete cure (RR 0.80, 95% CI 0.68 to 0.95; 1 RCT, 292 participants; high-certainty evidence). Compared to IMMA and placebo, at 26 weeks FU, adding oral pentoxifylline to IMMA to treat CL (species not stated) probably makes little to no difference to the likelihood of complete cure (RR 0.86, 95% CI 0.63 to 1.18; 1 RCT, 70 participants; moderate-certainty evidence).
Authors' conclusions: Evidence certainty was mostly moderate or low, due to methodological shortcomings, which precluded conclusive results. Overall, both IMMA and oral miltefosine probably result in an increase in cure rates, and nausea and vomiting are probably more common with miltefosine than with IMMA. Future trials should investigate interventions for mucosal leishmaniasis and evaluate recurrence rates of cutaneous leishmaniasis and its progression to mucosal disease.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Mariona Pinart: none known. José‐Ramón Rueda: none known. Gustavo AS Romero: author on included study Toledo 2014 but not involved in data extraction or assessment of risks of bias. Carlos Eduardo Pinzón‐Flórez: none known. Luz Karime Osorio Arango: none known. Ana Nilce Silveira Maia‐Elkhoury: none known. Ludovic Reveiz: none known. Ludovic Reveiz has contributed to this review in a personal capacity and during his spare time. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the organisation where he works. Vanessa M Elias: none known. John A Tweed: none known
Figures
























































































































Update of
-
Interventions for American cutaneous and mucocutaneous leishmaniasis.Cochrane Database Syst Rev. 2009 Apr 15;(2):CD004834. doi: 10.1002/14651858.CD004834.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD004834. doi: 10.1002/14651858.CD004834.pub3. PMID: 19370612 Updated.
References
References to studies included in this review
Almeida 1999 {published data only}
-
- Almeida R, D'Oliveira A Jr, Machado P, Bacellar O, Ko AI, Jesus AR, et al. Randomized, double-blind study of stibogluconate plus human granulocyte macrophage colony stimulating factor versus stibogluconate alone in the treatment of cutaneous leishmaniasis. Journal of Infectious Diseases 1999;180(5):1735-7. [CENTRAL: CN-00266553] - PubMed
Alves 2016 {published data only}
-
- Alves FHC, E Silva JSF, De Araujo Pereira LI, De Paula CDR, Ribeiro RN, Gomes CM, et al. The efficacy of pentamidine in comparison to pentavalent antimonial in American tegumentary leishmaniasis: an open label, randomized, controlled trial. Journal of the American Academy of Dermatology 2016;74(5 Suppl 1):AB155. [CENTRAL: CN-01160654]
Alves Noroes 2015 {published data only}
-
- Alves Norões IJ, Moraes MO, Silva Feitosa TT, Bonilha de Souza LS, Gonçalves Júnior J, Rolim-Neto ML, et al. Avaliation the therapeutic efficacy of fluconazol. International Archives of Medicine 2015;8(135):1-16. [DOI: 10.3823/1734] - DOI
-
- Lima da Silva CG. Avaliaçao da eficácia terapêutica do fluconazol na leishmaniose tegumentar humana [Doctoral hesis]. Faculdade de Medicina, Fortaleza, Brazil: Universidade Federal do Ceará, 2012.
Andersen 2005 {published data only}
-
- Andersen EM, Cruz-Saldarriaga M, Llanos-Cuentas A, Luz-Cjuno M, Echevarria J, Miranda-Verasategui C, et al. Comparison of meglumine and pentamidine for Peruvian cutaneous leishmaniasis. American Journal of Tropical Medicine and Hygiene 2005;72(2):133-7. [CENTRAL: CN-00502527] - PubMed
Arana 1994 {published data only}
-
- Arana BA, Navin TR, Arana FE, Berman JD, Rosenkaimer F. Efficacy of a short course (10 days) of high-dose meglumine antimoniate with or without Interferon-gamma in treating cutaneous leishmaniasis in Guatemala. Clinical Infectious Diseases 1994;18(3):381-4. [CENTRAL: CN-00102358] - PubMed
Arana 2001 {published data only}
-
- Arana BA, Mendoza CE, Rizzo NR, Kroeger A. Randomized, controlled, double-blind trial of topical treatment of cutaneous leishmaniasis with the paromomycin plus methylbenzethonium chloride ointment in Guatemala. American Journal of Tropical Medicine and Hygiene 2001;65(5):466-70. [CENTRAL: CN-00375557] - PubMed
Arévalo 2007 {published data only}
-
- Arévalo I, Tulliano G, Quispe A, Spaeth G, Mathlashewski, Llanos-cuantas A, et al. Role of imiquimod and parenteral meglumine antimoniate in the intitial treatment of cutaneous lesihmaniasis. Clinical Infectious Diseases 2007;44(12):1549-54. [CENTRAL: CN-00589061] - PubMed
Armijos 2004 {published data only}
-
- Armijos RX, Weigel MM, Calvopiña M, Mancheno M, Rodriguez R. Comparison of the effectiveness of two topical paromomycin treatments versus meglumine antimoniate for New World cutaneous leishmaniasis. Acta Tropica 2004;91(2):153-60. [CENTRAL: CN-00480899] - PubMed
Ballou 1987 {published data only}
-
- Ballou WR, McClain JB, Gordon DM, Dhanks GD, Adujar J, Berman JD, et al. Safety and efficacy of high-dose sodium stibogluconate therapy of American cutaneous leishmaniasis. Lancet 1987;4(8549):13-6. [CENTRAL: CN-00048758] - PubMed
Brito 2014 {published data only}
Brito 2017a {published data only}
Chico 1995 {published data only}
-
- Chico M, Armijos R, Racines J, Mancero T, Guderian RH. Evaluation of allopurinol-riboside therapy against Ecuadorian cutaneous leishmaniasis [Evaluación del ribósido de alpurinol contra la leishmaniasis cutánea ecuatoriana]. Biomedica 1995;15(3):116-22. [DOI: 10.7705/biomedica.v15i3.868] - DOI
Chrusciak‐Talhari 2011 {published data only}
-
- Chrusciak-Talhari A, Dietze R, Chrusciak Talhari C, Da Silva RM, Gadelha Yamashita EP, De Oliveira Penna G, et al. Randomized controlled clinical trial to access efficacy and safety of miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. American Journal of Tropical Medicine and Hygiene 2011;84(2):255-60. [CENTRAL: CN-00778313] - PMC - PubMed
Convit 1987 {published data only}
-
- Convit J, Castellanos PL, Rondon A, Pinardi ME, Ulrich M, Castes M, et al. Immunotherapy versus chemotherapy in localised cutaneous leishmaniasis. Lancet 1987;21(8530):401-5. [CENTRAL: CN-00046375] - PubMed
Convit 1989 {published data only}
-
- Convit J, Castellanos PL, Ulrich M, Castés M, Rondón A, Pinardi ME, et al. Immunotherapy of localized, intermediate, and diffuse forms of American cutaneous leishmaniasis. Journal of Infectious Diseases 1989;160(1):104-15. [CENTRAL: CN-00060507] - PubMed
Correia 1996 {published data only}
-
- Correia D, Macêdo VO, Carvalho EM, Barral A, Magalhâes AV, De Abreu MV, et al. Comparative study of meglumine antimoniate, pentamidine isothionate and aminosidine sulphate in the treatment of primary skin lesions caused by Leishmania (viannia) braziliensis. Revista da Sociedade Brasilera de Medicina Tropical 1996;29(5):447-53. [CENTRAL: CN-00132502] - PubMed
Cossio‐Duque 2015 {published data only}
-
- Cossio-Duque A, Mar CM, Navas A, Valderrama L, Cuervo-Pardo L, Marquez R, et al. Effect of the addition of pentoxifylline on the therapeutic and inflammatory response in patients with cutaneous leishmaniasis: a randomized placebo controlled trial. Frontiers 2015;93(4 Suppl):536. [CENTRAL: CN-01249729]
D'Oliveira 1997 {published data only}
-
- D'Oliveira A Jr, Machado PR, Carvalho EM. Evaluating the efficacy of allopurinol for treatment of cutaneous leishmaniasis. International Journal of Dermatology 1997;36(12):938-40. [CENTRAL: CN-00147570] - PubMed
Echevarria 2006 {published data only}
-
- Echevarria J, Seas C, Cruz M, Chavez E, Campos M, Cieza J, et al. Oral rehydration solution to prevent nephrotoxicity of amphotericin B. American Journal of Tropical Medicine and Hygiene 2006;75(6):1108-12. [CENTRAL: CN-00577315] - PubMed
Ferreira 2014 {unpublished data only}
-
- Ferreira Terceiro BR. Comparação entre o esquema padrão e alternativo de antimoniato de meglumina no tratamento da leishmaniose mucocutânea ou mucosa [Comparison of the standard scheme and alternative meglumine antimoniate in the treatment of leishmaniasis mucocutaneous or mucosal] [Masters thesis]. Rio de Janeiro: Instituto Nacional de Infectologia Evandro Chagas, 2014. [lil-751645]
Figueiredo 1999 {published data only}
-
- Figueiredo Kopke LF, Siviero do Vale EC, Grossi Araujo M, Araújo Magalhâes P, Furtado T. Treatment of American cutaneous leishmaniasis by N-methyl-glucamine. Double-blind study with dosages of 14 mg/kg/day and 28 mg/kg/day of antimony [Tratamento da leishmaniose tegumentar americana pelo antimoniato de N-metil-glucamina: Estudo duplo-cego com doses de 14 mg/kg/dia e 28 mg/kg/dia de antimônio]. Anais Brasileiros de Dermatologia 1991;66(2):87-94. [CENTRAL: CN-00187782] [EMBASE: 21169232]
Franke 1994 {published data only}
-
- Franke ED, Llanos-Cuentas A, Echevarria J, Cruz ME, Campos P, Tovar AA, et al. Efficacy of 28-day and 40-day regimens of sodium stibogluconate (pentostam) in the treatment of mucosal leishmaniasis. American Journal of Tropical Medicine and Hygiene 1994;51(1):77-82. [CENTRAL: CN-00103648] - PubMed
Gadelha 2018 {published data only}
-
- Gadelha EP, Ramasawmy R, Da Costa Oliveira B, Morais Rocha N, De Oliveira Guerra JA, Allan Villa Rouco da Silva G, et al. An open label randomized clinical trial comparing the safety and effectiveness of one, two or three weekly pentamidine isethionate doses (seven milligrams per kilogram) in the treatment of cutaneous leishmaniasis in the Amazon Region. PLOS Neglected Tropical Diseases 2018;12(10):e0006850. - PMC - PubMed
Garcia 2014 {published data only}
-
- Garcia Bustos MF, Barrio A, Parodi C, Beckar J, Moreno S, Basombrio MA. Miltefosine versus meglumine antimoniate in the treatment of mucosal leishmaniasis. Medicina 2014;74(5):371-7. [CENTRAL: CN-01089869] - PubMed
Guderian 1991 {published data only}
-
- Guderian RH, Chico ME, Rogers MD, Pattishall KM, Grogl M, Berman JD. Placebo controlled treatment of Ecuadorian cutaneous leishmaniasis. American Journal of Tropical Medicine and Hygiene 1991;45(1):92-7. [CENTRAL: CN-00077349] - PubMed
Guzman‐Rivero 2014 {published data only}
-
- Guzman-Rivero M, Rojas E, Verduguez-Orellana A, Pardo H, Torrico MC, Cloetens L, et al. Nutritional status in patients with cutaneous leishmaniasis and a study of the effects of zinc supplementation together with antimony treatment. Food & Nutrition Research 2014;58:23353. [CENTRAL: CN-01072233] [DOI: 10.3402/fnr.v58.23353] - DOI - PMC - PubMed
Hepburn 1994 {published data only}
-
- Hepburn NC, Tidman MJ, Hunter JA. Aminosidine (paromomycin) versus sodium stibogluconate for the treatment of American cutaneous leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(6):700-3. [CENTRAL: CN-00111782] - PubMed
Hu 2015 {published data only}
-
- Hu RV, Straetemans M, Kent AD, Sabajo LO, De Vries HJ, Fat RF. Randomized single-blinded non-inferiority trial of 7 mg/kg pentamidine isethionate versus 4 mg/kg pentamidine isethionate for cutaneous leishmaniaisis in Suriname. PLOS Neglected Tropical Diseases 2015;9(3):e0003592. [CENTRAL: CN-01256791] - PMC - PubMed
Krolewiecki 2007 {published data only}
-
- Krolewiecki AJ, Romero HD, Cajal SP, Abraham D, Mimori T, Matsumoto T, et al. A randomized clinical trial comparing oral azithromycin and meglumine antimoniate for the treatment of American cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis. American Journal of Tropical Medicine and Hygiene 2007;77(4):640-6. [CENTRAL: CN-00620647] - PubMed
Llanos‐Cuentas 1997 {published data only}
-
- Llanos-Cuentas A, Echevarria J, Cruz M, La Rosa A, Campos P, Campos M, et al. Efficacy of sodium stibogluconate alone and in combination with allopurinol for treatment of mucocutaneous leishmaniasis. Clinical Infectious Diseases 1997;25(3):677-84. [CENTRAL: CN-00143908] - PubMed
Llanos‐Cuentas 2007 {published data only}
-
- Llanos-Cuentas A, Echevarria J, Seas C, Chang E, Cruz M, Alvarez E, et al. Parenteral aminosidine is not effective for peruvian mucocutaneous leishmaniasis. American Journal of Tropical Medicine and Hygiene 2007;76(6):1128-31. [CENTRAL: CN-00703926] - PubMed
Lobo 2006 {published data only}
-
- Lobo IM, Soares MB, Correia TM, De Freitas LA, Oliveira MI, Nakatani M, et al. Heat therapy for cutaneous leishmaniasis elicits a systemic cytokine response similar to that of antimonial (Glucantime) therapy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2006;100(7):642-9. [CENTRAL: CN-00561435] - PubMed
López 2018 {published data only}
-
- López L, Vélez I, Asela C, Cruz C, Alves F, Robledo S, et al. A phase II study to evaluate the safety and efficacy of topical 3% amphotericin B cream (Anfoleish) for the treatment of uncomplicated cutaneous leishmaniasis in Colombia. PLOS Neglected Tropical Disiseases 2018;12(7):e0006653. - PMC - PubMed
Lopez‐Jaramillo 2010 {published data only}
-
- Lopez-Jaramillo P, Rincon MY, Garcia RG, Silva SY, Smith E, Kampeerapappun P, et al. A controlled, randomized-blinded clinical trial to assess the efficacy of a nitric oxide releasing patch in the treatment of cutaneous leishmaniasis by Leishmania (V.) panamensis. American Journal of Tropical Medicine and Hygiene 2010;83(1):97-101. [CENTRAL: CN-00749347] - PMC - PubMed
Machado 2007 {published data only}
-
- Machado PR, Lessa H, Lessa M, Guimaraes LH, Bang H, Ho JL, et al. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clinical Infectious Diseases 2007;44(6):788-93. [CENTRAL: CN-00578520] - PubMed
Machado 2010 {published data only}
-
- Machado PR, Ampuero J, Guimaraes LH, Schriefer A, Carvalho EM, Talhari S, et al. Efficacy of miltefosine in the treatment of American cutaneous leishmaniasis caused by leishmania braziliensis in Brazil. PLOS Neglected Tropical Diseases 2010;4(12):e912. [DOI: 10.4269/ajtmh.2009.81.201] - DOI - PMC - PubMed
Machado 2018 {published data only}
-
- Machado PR, Ribeiro CS, França-Costa J, Dourado ME, Trinconi CT, Yokoyama-Yasunaka JK, et al. Tamoxifen and meglumine antimoniate combined therapy in cutaneous leishmaniasis patients: a randomised trial. Tropical Medicine & International Health 2018;23(9):936-42. - PubMed
Machado‐Pinto 2002 {published data only}
-
- Machado-Pinto J, Pinto J, Da Costa CA, Genaro O, Marques MJ, Modabber F, et al. Immunochemotherapy for cutaneous leishmaniasis: a controlled trial using killed Leishmania (Leishmania) amazonensis vaccine plus antimonial. International Journal of Dermatology 2002;41(2):73-8. [CENTRAL: CN-00389013] - PubMed
Martínez 1992 {published data only}
-
- Martínez S, Marr J. Allopurinol in the treatment of American cutaneous leishmaniasis. New England Journal of Medicine 1992;326(11):741-4. [CENTRAL: CN-00081583] - PubMed
Martínez 1997 {published data only}
-
- Martínez S, Gonzalez M, Vernaza ME. Treatment of cutaneous leishmaniasis with allopurinol and stibogluconate. Clinical Infectious Diseases 1997;24(2):165-9. [CENTRAL: CN-00138905] - PubMed
Miranda‐Verástegui 2005 {published data only}
-
- Miranda-Verástegui C, Llanos-Cuentas A, Arévalo I, Ward BJ, Matlashewski G. Randomized, double-blind clinical trial of topical imiquimod 5% with parenteral meglumine antimoniate in the treatment of cutaneous leishmaniasis in Peru. Clinical Infectious Diseases 2005;40(10):1395-403. [CENTRAL: CN-00560657] - PubMed
Miranda‐Verástegui 2009 {published data only}
-
- Miranda-Verástegui C, Tulliano G, Gyorkos TW, Calderon W, Rahme E, Ward B, et al. First-line therapy for human cutaneous leishmaniasis in Peru using the TLR7 agonist imiquimod in combination with pentavalent antimony. PLOS Neglected Tropical Diseases 2009;3(7):e491. [CENTRAL: CN-00729032] - PMC - PubMed
Navin 1990 {published data only}
-
- Navin TR, Arana BA, Arana FE, De Mérida AM, Castillo AL, Pozuelos JL. Placebo-controlled clinical trial of meglumine antimoniate (glucantime) versus localized controlled heat in the treatment of cutaneous leishmaniasis in Guatemala. American Journal of Tropical Medicine and Hygiene 1990;42(1):43-50. [CENTRAL: CN-00065499] - PubMed
Navin 1992 {published data only}
-
- Navin TR, Aran BA, Arana FE, Berman JD, Chajón JF. Placebo-controlled clinical trial of sodium stibogluconate (pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. Journal of Infectious Diseases 1992;165(3):528-34. [CENTRAL: CN-00081938] - PubMed
NCT01011309 {published data only}
-
- NCT01011309. A study of the efficacy and safety of the LEISH-F2 + MPL-SE vaccine for treatment of cutaneous leishmaniasis [A phase 2, randomized, open-label, controlled study to evaluate the efficacy, safety, and immunogenicity of the LEISH-F2 + MPL-SE vaccine in the treatment of patients with cutaneous leishmaniasis]. clinicaltrials.gov/ct2/show/study/NCT01011309 (first received 11 November 2009).
Neva 1997 {published data only}
-
- Neva FA, Ponce C, Ponce E, Kreutzer R, Modabber F, Olliaro P. Non-ulcerative cutaneous leishmaniasis in Honduras fails to respond to topical paromomycin. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(4):473-5. [CENTRAL: CN-00145412] - PubMed
Neves 2011 {published data only}
-
- Neves LO, Talhari AC, Gadelha EP, Silva Junior RM, Guerra JA, Ferreira LC, et al. A randomized clinical trial comparing meglumine antimoniate, pentamidine and amphotericin B for the treatment of cutaneous leishmaniasis by Leishmania guyanensis. Anais Brasileiros de Dermatologia 2011;86(6):1092-101. [CENTRAL: CN-00842795] - PubMed
Newlove 2011 {published data only}
-
- Newlove T, Guimaraes LH, Morgan DJ, Alcantara L, Glesby MJ, Carvalho EM, et al. Antihelminthic therapy and antimony in cutaneous leishmaniasis: a randomized, double-blind, placebo-controlled trial in patients co-infected with helminths and Leishmania braziliensis. American Journal of Tropical Medicine and Hygiene 2011;84(4):551-5. [CENTRAL: CN-00788444] - PMC - PubMed
Oliveira‐Neto 1997 {published data only}
-
- Oliveira-Neto MP, Schubach A, Mattos M, Gonçalves-Costa SC, Pirmez C. Treatment of American cutaneous leishmaniasis: a comparison between low dosage (5mg/kg/day) and high dosage (20 mg/kg/day) antimony regimens [Traitement de la leishmanoise cutanee americaine: une comparison entre faible dose (5mg/kg/jour) et haute dose (20mg/kg/jour) d'antimoine]. Pathologie-biologie 1997;45(6):496-9. [CENTRAL: CN-00254046] - PubMed
Oster 1985 {published data only}
-
- Oster CN, Chulay JD, Hendricks LD, Pamplin CL III, Ballou WR, Berman JD, et al. American cutaneous leishmaniasis: a comparison of three sodium stibogluconate treatment schedules. American Journal of Tropical Medicine and Hygiene 1985;34(5):856-60. [CENTRAL: CN-00039556] - PubMed
Palacios 2001 {published data only}
-
- Palacios R, Osorio LE, Grajales LF, Ochoa MT. Treatment failure in children in a randomized clinical trial with 10 and 20 days of meglumine antimoniate for cutaneous leishmaniasis due to leishmania viannia species. American Journal of Tropical Medicine and Hygiene 2001;64(3-4):187-93. [CENTRAL: CN-00349275] - PubMed
Prates 2017 {published data only}
-
- Prates FV, Dourado ME, Silva SC, Schriefer A, Guimarães LH, Brito MD, et al. Fluconazole in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis: a randomized controlled trial. Clinical Infectious Diseases 2017;64(1):67-71. [CENTRAL: CN-01444134] - PubMed
Ravis 2013 {published data only}
-
- Ravis WR, Llanos-Cuentas A, Sosa N, Kreishman-Deitrick M, Kopydlowski KM, Nielsen C, et al. Pharmacokinetics and absorption of paromomycin and gentamicin from topical creams used to treat cutaneous leishmaniasis. Antimicrobial Agents and Chemotherapy 2013;57(10):4809-15. [CENTRAL: CN-00916363] - PMC - PubMed
Rubiano 2012 {published data only}
Saenz 1987 {published data only}
-
- Saenz RE, Paz HM, Johnson CM, Narvaez E, De Vasquez AM. Evaluation of the effectiveness and toxicity of pentostam and glucantime in the treatment of cutaneous leishmaniasis [Evaluación de la effectividad y toxicidad del pentostam y del glucantime en el tratamiento de la leishmaniasis cutanea]. Revista Médica de Panamá 1987;12(3):148-57. [CENTRAL: CN-01139764] - PubMed
Saenz 1990 {published data only}
-
- Saenz RE, Paz H, Berman JD. Efficacy of ketoconazole against leishmania braziliensis panamensis cutaneous leishmaniasis. American Journal of Medicine 1990;89(2):147-55. [CENTRAL: CN-00069419] - PubMed
Saheki 2017 {published data only}
-
- Lyra MR. Phase III clinical trial for American cutaneous leishmaniasis. Equivalence between high and low dose schemes of meglumine antimoniate [Masters thesis] [Ensaio clínico fase iii para leishmaniose tegumentar americana forma cutânea. Equivalência entre esquemas de alta e baixa dose de antimoniato de meglumina]. www.arca.fiocruz.br/handle/icict/14378 (accessed prior to 1 February 2019).
-
- Ribeiro MN, Pimentel MI, Schubach Ade O, Oliveira R de V, Teixeira JL, Leite MP, et al. Factors associated with adherence to different treatment schemes with meglumine antimoniate in a clinical trial for cutaneous leishmaniasis. Revista do Instituto de Medicina Tropical de Sao Paulo 2014;56(4):291-6. - PMC - PubMed
-
- Saheki MN, Lyra MR, Bedoya-Pacheco SJ, Antonio LF, Pimentel MIF, Salgueiro MM, et al. Low versus high dose of antimony for American cutaneous leishmaniasis: a randomized controlled blind non-inferiority trial in Rio de Janeiro, Brazil. PLOS One 2017;12(5):e0178592. [CENTRAL: CN-01413190] - PMC - PubMed
-
- Saheki MN. A randomized, controlled, single blind, non-inferiority trial of low dose versus high dose meglumine antimoniate for American cutaneous leishmaniasis in Rio de Janeiro, Brazil [Masters thesis] [Ensaio clínico de não inferioridade, randomizado, controlado, cego,de antimoniato de meglumina em dose baixa versus dose alta paraleishmaniose cutânea americana no Rio de Janeiro, Brasil]. www.arca.fiocruz.br/handle/icict/12458 (accessed prior to 1 February 2019).
Sampaio 2019 {published data only}
-
- Sampaio RN, Silva Jsfe, Paula CD, Porto C, Motta Jocd, Pereira LI, et al. A randomized, open-label clinical trial comparing the long-term effects of miltefosine and meglumine antimoniate for mucosal leishmaniasis. Revista da Sociedade Brasileira de Medicina Tropical 2019;52:e20180292. - PubMed
Santos 2004 {published data only}
-
- Santos JB, De Jesus AR, Machado PR, Magalhâes A, Salgado K, Carvalho EM, et al. Antimony plus recombinant human granulocyte-macrophage colony-stimulating factor applied topically in low doses enhances healing of cutaneous leishmaniasis ulcers: a randomized, double-blind, placebo-controlled study. Journal of Infectious Diseases 2004;190(10):1793-6. [CENTRAL: CN-00492355] - PubMed
Sosa 2013 {published data only}
Sosa 2019 {published data only}
-
- NCT01790659. Phase 3 study of Walter Reed (WR) 279,396 and paromomycin alone for the treatment of cutaneous leishmaniasis in Panama [A randomized, double-blind, pivotal phase 3 study of WR 279,396 (paromomycin + gentamicin topical cream) and paromomycin alone topical cream for the treatment of cutaneous leishmaniasis in Panama]. clinicaltrials.gov/ct2/show/study/NCT01790659 (first received 13 February 2013).
Soto 1994a {published data only}
-
- Soto J, Grogl M, Berman J, Olliaro P. Limited efficacy of injectable aminosidine as single-agent therapy for Colombian cutaneous leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(6):695-8. [CENTRAL: CN-00111780] - PubMed
Soto 1998 {published data only}
-
- Soto J, Fuya P, Herrera R, Berman J. Topical paromomycin/methylbenzethonium chloride plus parenteral meglumine antimoniate as treatment for American cutaneous leishmaniasis: controlled study. Clinical Infectious Diseases 1998;26(1):56-8. [CENTRAL: CN-00147306] - PubMed
Soto 2002 {published data only}
-
- Soto JM, Toledo JT, Gutierrez P, Arboleda M, Nicholls RS, Padilla JR, et al. Treatment of cutaneous leishmaniasis with a topical antileishmanial drug (WR279396): phase 2 pilot study. American Journal of Tropical Medicine and Hygiene 2002;66(2):147-51. [CENTRAL: CN-00390421] - PubMed
Soto 2004a {published data only}
-
- Soto J, Valda-Rodriguez L, Toledo J, Vera-Navarro L, Luz M, Monasterios-Torrico H, et al. Comparison of generic to branded pentavalent antimony for treatment of new world cutaneous leishmaniasis. American Journal of Tropical Medicine and Hygiene 2004;71(5):577-81. [CENTRAL: CN-00504004] - PubMed
Soto 2004b {published data only}
-
- Soto J, Arana BA, Toledo J, Rizzo N, Vega JC, Diaz A, et al. Miltefosine for new world cutaneous leishmaniasis. Clinical Infectious Diseases 2004;38(9):1266-72. [CENTRAL: CN-00470183] - PubMed
Soto 2008 {published data only}
-
- Soto J, Rea J, Balderrama M, Toledo J, Soto P, Valda L, et al. Efficacy of miltefosine for Bolivian cutaneous leishmaniasis. American Journal of Tropical Medicine and Hygiene 2008;78(2):210-1. [CENTRAL: CN-00629911] - PubMed
Soto 2013 {published data only}
-
- Soto J, Rojas E, Guzman M, Verduguez A, Nena W, Maldonado M, et al. Intralesional antimony for single lesions of Bolivian cutaneous leishmaniasis. Clinical Infectious Diseases 2013;56(9):1255-60. [CENTRAL: CN-00877427] - PubMed
Soto 2016a {published data only}
Soto 2016b {published data only}
Soto 2019 {published data only}
-
- Soto J, Soto P, Ajata A, Luque C, Tintaya C, Paz D, et al. Topical 15% paromomycin-aquaphilic for Bolivian leishmania braziliensis cutaneous leishmaniasis: a randomized, placebo-controlled trial. Clinical Infectious Diseases 2019;68(5):844-9. - PubMed
Souza 1998 {published data only}
-
- Souza e Souza I, Lima IC, Alecrim JF. American cutaneous leishmaniasis: therapeutic study of pentamidine isotionate versus N-metil-glucantime. Journal of the European Academy of Dermatology and Venereology : JEADV 1998;11(Suppl 2):S153.
Toledo 2014 {published data only}
-
- Toledo Junior A, Daher AB, Amaral TA, Carvalho SF, Romero GA, Rabello A. Poor response to azithromycin in cutaneous leishmaniasis leading to a premature interruption of a multicentric phase III clinical trial in Brazil. Revista Da Sociedade Brasileira de Medicina Tropical 2014;47(6):756-62. [CENTRAL: CN-01048573] - PubMed
Vélez 1997 {published data only}
-
- Vélez I, Agudelo S, Hendrickx E, Puerta J, Grogl M, Modabber F, et al. Inefficacy of allopurinol as monotherapy for Colombian cutaneous leishmaniasis: a randomized, controlled trial. Annals of Internal Medicine 1997;126(3):232-6. [CENTRAL: CN-00135976] - PubMed
Vélez 2010 {published data only}
-
- López L, Cruz C, Godoy G, Robledo SM, Vélez ID. Thermotherapy effective and safer than miltefosine in the treatment of cutaneous leishmaniasis in Colombia. Revista do Instituto de Medicina Tropical de Sao Paulo 2013;55(3):S0036-46652013000300197. - PubMed
References to studies excluded from this review
Armijos 2004B {published data only}
-
- Armijos RX, Weigel MM, Calvopina M, Hidalgo A, Cevallos W, Correa J. Safety, immunogeneicity, and efficacy of an autoclaved leishmania amazonensis vaccine plus BCG adjuvant against New World cutaneous leishmaniasis. Vaccine 2004;22(9-10):1320-6. [CENTRAL: CN-00471149] - PubMed
De Luca 1999 {published data only}
-
- De Luca PM, Mayrink W, Alves CR, Coutinho SG, Oliveira MP, Bertho AL, et al. Evaluation of the stability and immunogenicity of autoclaved and nonautoclaved preparations of a vaccine against American tegumentary leishmaniasis. Vaccine 1999;17(9-10):1179-85. [CENTRAL: CN-00305330] - PubMed
De Luca 2001 {published data only}
-
- De Luca PM, Mayrink W, Pinto JA, Coutinho SG, Santiago MA, Toledo VP, et al. A randomized double-blind placebo-controlled trial to evaluate the immunogenicity of a candidate vaccine against American tegumentary leishmaniasis. Acta Tropica 2001;80(3):251-60. [CENTRAL: CN-00375249] - PubMed
De Luca 2003 {published data only}
-
- De Luca PM, Mayrink W, Santiago MA, Nogueira R, Conceição-Silva F, Mélo G, et al. Randomized, double-blind, placebo-controlled study on the immunogenicity of the leishmanin skin test. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(6):709-12. [CENTRAL: CN-00551996] - PubMed
Deps 2000 {published data only}
-
- Deps PD, Viana MC, Falqueto A, Dietze R. Evaluation of the efficacy and toxicity of N-methyl-glucamine vs BP88 Sodium Stibogluconate in the treatment of localized cutaneous leishmaniasis. Revista da Sociedade Brasileira de Medicina Tropical 2000;33(6):535-43. [CENTRAL: CN-00399871] - PubMed
Fagundes 2007 {published data only}
-
- Fagundes A, Marzochi MC, Perez M, Schubach A, Ferreira A, Silva JP, et al. Skin reactivity to thimerosal and phenol-preserved Montenegro antigen in Brazil. Acta Tropica 2007;101(1):25-30. [CENTRAL: CN-00577747] - PubMed
Falquete 2002 {published data only}
-
- Falquete A, Sessa AP. Leishmaniose tegumentar americana. In: Veronesi R, Focaccia R, editors(s). Tratado de infectologia. Sâo Paulo: Atheneu, 2002:1241-53.
Gardlo 2003 {published data only}
-
- Gardlo K, Horska Z, Enk CD, Rauch L, Megahed M, Ruzicka T, et al. Treatment of cutaneous leishmaniasis by photodynamic therapy. Journal of the American Academy of Dermatology 2003;48(6):893-6. [CENTRAL: CN-00466020] - PubMed
Hendrickx 1998 {published data only}
-
- Hendrickx EP, Agudelo SP, Munoz DL, Puerta JA, Velez Bernal ID. Lack of efficacy of mefloquine in the treatment of New World cutaneous leishmaniasis in Colombia. American Journal of Tropical Medicine and Hygiene 1998;59(6):889-92. [PMID: ] - PubMed
Hepburn 1993 {published data only}
-
- Hepburn NC, Tidman MJ, Hunter JA. Cutaneous leishmaniasis in British troops from Belize. British Journal of Dermatology 1993;128(1):63-8. [PMID: ] - PubMed
Hepburn 1994b {published data only}
-
- Hepburn NC, Siddique I, Howie AF, Beckett GJ, Hayes PC. Hepatotoxicity of sodium stibogluconate therapy for American cutaneous leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(4):453-5. [CENTRAL: CN-00119186] - PubMed
Herwaldt 1992 {published data only}
-
- Herwaldt BL, Arana BA, Navin TR. The natural history of cutaneous leishmaniasis in Guatemala. Journal of Infectious Diseases 1992;165(3):518-27. [PMID: ] - PubMed
Krause 1999 {published data only}
-
- Krause G, Kroeger A. Topical paromomycin/methylbenzethonium chloride plus parenteral meglumine antimonate as treatment of American cutaneous leishmaniasis: controlled study. Clinical Infectious Diseases 1999;29(2):466-7. [PMID: ] - PubMed
Laguna‐Torres 1999 {published data only}
-
- Laguna-Torres VA, Silva CA, Correia D, Carvalho EM, Magalhâes AV, De Oliveira Macêdo V. Efficacy of mefloquine in the treatment of skin leishmaniasis in an endemic area of leishmania (viannia) braziliensis. Revista da Sociedade Brasileira de Medicina Tropical 1999;32(5):529-32. [CENTRAL: CN-00414710] - PubMed
Llanos 1991 {published data only}
-
- Llanos A, Cieza J, Bernardo J, Echevarria J, Biaggioni I, Sabra R, et al. Effect of salt supplementation on amphotericin B nephrotoxicity. Kidney International 1991;40(2):302-8. [CENTRAL: CN-00079305] - PubMed
Llanos‐Cuentas 2010 {published data only}
-
- Llanos-Cuentas A, Calderon W, Cruz M, Ashman JA, Alves FP, Coler RN, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with sodium stibogluconate for the treatment of mucosal leishmaniasis. Vaccine 2010;28(46):7427-35. [CENTRAL: CN-00768896] - PubMed
Monjour 1994 {published data only}
-
- Monjour L, Neogy AB, Vouldoukis I, Silva OA, Boisnic S, Brito ME, et al. Exploitation of parasite derived antigen in therapeutic success of human cutaneous leishmaniasis in Brazil. Memórias do Instituto Oswaldo Cruz 1994;89(3):479-83. [CENTRAL: CN-00118323] - PubMed
Motta 2012 {published data only}
-
- Motta JO, Sampaio RN. A pilot study comparing low-dose liposomal amphotericin B with N-methyl glucamine for the treatment of American cutaneous leishmaniasis. Journal of the European Academy of Dermatology and Venereology : JEADV 2012;26(3):331-5. [CENTRAL: CN-00882917] - PubMed
Nascimento 1990 {published data only}
Oliveira‐Neto 2000 {published data only}
-
- Oliveira-Neto MP, Mattos M, Primez C, Fernandes O, Gonçalves-Costa SC, Souza CF, et al. Mucosal leishmaniasis ("espundia") responsive to low dose of N-methyl glucantime in Rio de Janeiro, Brazil. Revista do Instituto de Medicina Tropical de São Paulo 2000;42(6):321-5. [CENTRAL: CN-00372731] - PubMed
Rodriguez 1995 {published data only}
-
- Rodriguez LV, Dedet JP, Paredes V, Mendoza C, Cardenas F. A randomized trial of amphotericin B alone or in combination with itraconazole in the treatment of mucocutaneous leishmaniasis. Memórias do Instituto Oswaldo Cruz 1995;90(4):525-8. [CENTRAL: CN-00121054] - PubMed
Saldanha 2000 {published data only}
-
- Saldanha AC, Romero GA, Guerra C, Merchan-Hamann E, Macedo Vde O. Comparative study between sodium stibogluconate BP 88 and meglumine antimoniate in cutaneous leishmaniasis treatment. II. Biochemical and cardiac toxicity. Revista da Sociedade Brasileira de Medicina Tropical 2000;33(4):383-8. [PMID: ] - PubMed
Soto 1994b {published data only}
-
- Soto J, Buffet P, Grogl M, Berman J. Successful treatment of Colombian cutaneous leishmaniasis with four injections of pentamidine. American Journal of Tropical Medicine and Hygiene 1994;50(1):107-11. [CENTRAL: CN-00098861] - PubMed
Soto 1995 {published data only}
-
- Soto J, Hernandez N, Mejia H, Grogl M, Berman J. Successful treatment of New World cutaneous leishmaniasis with a combination of topical paromomycin/methylbenzethonium chloride and injectable meglumine antimonate. Clinical Infectious Diseases 1995;20(1):47-51. [CENTRAL: CN-00113359] - PubMed
Soto‐Mancipe 1993 {published data only}
-
- Soto-Mancipe J, Grogl M, Berman JD. Evaluation of pentamidine for the treatment of cutaneous leishmaniasis in Colombia. Clinical Infectious Diseases 1993;16(3):417-25. [CENTRAL: CN-00091785] - PubMed
Urcuyo 1982 {published data only}
-
- Urcuyo FG, Zaias N. Oral ketoconazole in the treatment of leishmaniasis. International Journal of Dermatology 1982;21(7):414-6. [PMID: ] - PubMed
Veiga 1985 {published data only}
-
- Veiga JP, Rosa TT, Kimachi T, Wolff ER, Sampaio RN, Gagliardi AR, et al. [Renal function in patients with mucocutaneous leishmaniasis treated with pentavalent antimony compounds]. Revista do Instituto de Medicina Tropical de Sao Paulo 1985;27(6):298-302. [PMID: ] - PubMed
Vélez 2005 {published data only}
-
- Vélez ID, Gilchrist K, Arbelaez MP, Rojas CA, Puerta JA, Antunes CM, et al. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Transactions of the Royal Society of Tropical Medicine and Hygiene 2005;99(8):593-8. [CENTRAL: CN-00521907] - PubMed
Wortmann 2002 {published data only}
-
- Wortmann G, Scoot Miller R, Oster C, Jackson J, Aronson N. A randomized, double-blind study of the efficacy of a 10- or 20-day course of sodium stibogluconate for treatment of cutaneous leishmaniasis in United States military personnel. Clinical Infectious Diseases 2002;35(3):261-7. [PMID: ] - PubMed
References to studies awaiting assessment
NCT00004755 {published data only}
-
- NCT00004755. Allopurinol, glucantime, or allopurinol/glucantime for cutaneous leishmaniasis in Brazil [Phase II randomized study of allopurinol versus glucantime versus allopurinol/glucantime for cutaneous leishmaniasis in Brazil]. clinicaltrials.gov/ct2/show/record/NCT00004755 (first received 25 February 2000).
NCT00111514 {published data only}
-
- NCT00111514. Study to evaluate the Leish-111F + MPL-SE vaccine in the treatment of mucosal leishmaniasis [A phase 1, randomized, double-blind, placebo-controlled, dose-escalating study to evaluate safety, tolerability, and immunogenicity of Leish-111f + MPL-SE vaccine in combination with pentavalent antimony in treatment of mucosal leishmaniasis]. clinicaltrials.gov/ct2/show/study/NCT00111514 (first received 23 May 2005).
NCT00111553 {published data only}
-
- NCT00111553. Study to evaluate the Leish-111F + MPL-SE vaccine in the treatment of cutaneous leishmaniasis [Randomized, double-blind, adjuvant- and placebo-controlled, dose-escalating study to evaluate safety, tolerability, and immunogenicity of Leish-111f + MPL-SE vaccine with meglumine antimoniate (glucantime) in cutaneous leishmaniasis]. clinicaltrials.gov/ct2/show/study/NCT00111553 (first received 24 May 2005).
NCT00317980 {published data only}
-
- NCT00317980. Safety and efficacy of low-dose pentavalent antimony for treatment of cutaneous leishmaniasis (Lowdosesb) [Phase IV randomized controlled clinical trial to evaluate the safety and efficacy of low-dose pentavalent antimony compared to the standard dose in patients with cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis]. clinicaltrials.gov/ct2/show/study/NCT00317980 (first received 25 April 2006).
NCT00973128 {published data only}
-
- NCT0973128. Reduced doses of antimony plus ranulocyte monocyte colony stimulating factor (GM-CSF) for cutaneous leishmaniasis (GMCSFSbv) [Reduced doses of antimony plus recombinant human GM-CSF compared with antimony in standard doses for cutaneous leishmaniasis: a randomized, single-blind, placebo-controlled, pilot study]. clinicaltrials.gov/ct2/show/study/NCT0973128 (first received 9 September 2009).
NCT01380301 {published data only}
-
- NCT01380301. Treatment of cutaneous leishmaniasis with a combination of miltefosine and antimony [Treatment of Bolivian cutaneous leishmaniasis with a combination of short courses of miltefosine and antimony]. clinicaltrials.gov/ct2/show/study/NCT01380301 (first received 27 June 2011).
NCT01380314 {published data only}
-
- NCT01380314. Oral miltefosine plus topical imiquimod to treat cutaneous leishmaniasis [Treatment of Bolivian cutaneous leishmaniasis with a combination of oral miltefosine plus topical imiquimod 5%]. clinicaltrials.gov/ct2/show/record/NCT01380314 (first received 27 June 2011).
NCT01464242 {published data only}
-
- NCT01464242. Add-on study of pentoxifylline in cutaneous leishmaniasis (GT) [Therapeutic gain of adding the immunomodulator pentoxifylline to the treatment of cutaneous leishmaniasis]. clinicaltrials.gov/ct2/show/record/NCT01464242 (first received 3 November 2011).
NCT03294161 {published data only}
-
- NCT03294161. Fourth-generation immucillin derivative DI4G associated therapy in cutaneous leishmaniasis [Transition-state analog inhibitor of human purine nucleoside phosphorylase as an adjunct in cutaneous leishmaniasis therapy: a randomized and controlled trial]. clinicaltrials.gov/ct2/show/study/NCT03294161 (first received 26 September 2017).
Silva 2006 {published data only}
-
- Silva SY, Rueda LC, Lopez M, Velez ID, Rueda-Clausen CF, Smith DJ, et al. Double blind, randomized controlled trial, to evaluate the effectiveness of a controlled nitric oxide releasing patch versus meglumine antimoniate in the treatment of cutaneous leishmaniasis [NCT00317629]. Trials 2006;7:14. [PMID: ] - PMC - PubMed
References to ongoing studies
NCT00537953 {published data only}
-
- NCT00537953. Short course of miltefosine and antimony to treat cutaneous leishmaniasis in Bolivia [Efficacy and safety of a short course of the combination of miltefosine and antimony to treat cutaneous leishmaniasis in Bolivia]. clinicaltrials.gov/ct2/show/study/NCT00537953 (first received 2 October 2007).
NCT01301937 {published data only}
-
- NCT01301937. Low antimonial dosage in American mucosal leishmaniasis [Phase III clinical trial for mucosal or mucocutaneous leishmaniasis. Comparison between the standard and alternative antimonial schemes]. clinicaltrials.gov/ct2/show/study/NCT01301937 (first received 23 February 2011).
NCT02530697 {published data only}
-
- NCT02530697. The association of miltefosine and pentoxifylline to treat mucosal leishmaniasis: a clinical trial in Brazil. clinicaltrials.gov/ct2/show/study/NCT02530697 (first received 21 August 2015).
NCT02687971 {published data only}
-
- NCT02687971. Thermotherapy + a short course of miltefosine for the treatment of uncomplicated cutaneous leishmaniasis in the New World. clinicaltrials.gov/ct2/show/study/NCT02687971 (first received 23 February 2016).
NCT03023111 {published data only}
-
- NCT03023111. Miltefosine and GM-CSF in cutaneous leishmaniasis: a randomized and controlled trial. clinicaltrials.gov/ct2/show/NCT03023111 (first received 18 January 2017).
NCT03084952 {published data only}
-
- NCT03084952. Phase 2 trial to evaluate 18-methoxycoronaridine efficacy, safety and tolerability in cutaneous leishmaniasis patients. clinicaltrials.gov/ct2/show/study/NCT03084952 (first received 21 March 2017).
NCT03829917 {published data only}
-
- NCT03829917. Oral miltefosine plus topical paromomycin In American cutaneous leishmaniasis. clinicaltrials.gov/show/nct03829917 (first received 1 August 2019).
NCT04072874 {published data only}
-
- NCT04072874. Evaluation of the safety and clinical activity of Curaleish lotion and cream in the topical treatment of cutaneous leishmaniasis in Colombia. clinicaltrials.gov/ct2/show/NCT04072874 (first received 4 September 2019).
NTR2076 {published data only}
-
- NTR2076. Treatment of cutaneous leishmaniasis with pentamidine isethionate in Suriname; a comparison study between two treatment regimes, 3 days vs 7 days [Clinical, parasitological and pharmaco-economical evaluation of a 3 days versus 7 days pentamidine isethionate regimen for cutaneous leishmaniasis in Suriname]. www.trialregister.nl/trialreg/admin/rctview.asp?TC=2076 (first received 25 September 2009).
PER‐007‐16 {published data only}
-
- PER-007-16. A randomized, open label multicenter study to determine the efficacy and safety of combining thermotherapy and a short course of miltefosine for the treatment of uncomplicated cutaneous leishmaniasis in the New World. www.ins.gob.pe/ensayosclinicos/rpec/recuperarECPBNuevo.asp?ver=EN&nu... (first received 5 July 2016).
RBR‐5r93wn {published data only}
-
- RBR-5r93wn. Comparison of the effect and toxicity between two options for the treatment of Mucosal Leishmaniasis: miltefosin and Liposomal Anfotericin B. apps.who.int/trialsearch/Trial2.aspx?TrialID=RBR-5r93wn (first received 15 January 2018).
RBR‐6mk5n4 {published data only}
-
- RBR-6mk5n4. Multicentre study evaluating the efficacy and safety of Intralesional administration of Meglumine Antimoniate compared to systemic treatment for cutaneous leishmaniasis. www.ensaiosclinicos.gov.br/rg/RBR-6mk5n4/ (first received 8 November 2017).
Additional references
Abdeladhim 2014
Alcais 1997
-
- Alcais A, Abel L, David C, Torrez ME, Flandre P, Dedet JP. Risk factors for onset of cutaneous and mucocutaneous leishmaniasis in Bolivia. American Journal of Tropical Medicine and Hygiene 1997;57(1):79-84. - PubMed
Alexander 2009
-
- Alexander B, Agudelo LA, Navarro JF, Ruiz J F, Molina J, Aguilera G, et al. Relationship between coffee cultivation practices in Colombia and exposure to infection with Leishmania. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(12):1263-8. - PubMed
Almeida 2005
-
- Almeida RP, Brito J, Machado PL, De Jesus AR, Schriefer A, Guimaraes LH, et al. Successful treatment of refractory cutaneous leishmaniasis with GM-CSF and antimonials. American Journal of Tropical Medicine and Hygiene 2005;73(1):79-81. - PubMed
Almeida 2011
-
- Almeida OL, Santos JB. Advances in the treatment of cutaneous leishmaniasis in the new world in the last ten years: a systematic literature review. Anais Brasileiros de Dermatologia 2011;86(3):497-506. - PubMed
Alvar 2012
Alvar 2013
-
- Alvar J, Croft S L, Kaye P, Khamesipour A, Sundar S, Reed SG. Case study for a vaccine against leishmaniasis. Vaccine 2013;31(Suppl 2):B244-9. - PubMed
Amato 2007
-
- Amato VS, Tuon FF, Siqueira AM, Nicodemo AC, Neto VA. Treatment of mucosal leishmaniasis in Latin America: systematic review. American Journal of Tropical Medicine and Hygiene 2007;77(2):266-74. - PubMed
Ampuero 2006
-
- Ampuero J, Macedo V, Marsden P. Clinical findings of tegumentary leishmaniasis in children under five years of age in an endemic area of Leishmania (Viannia) braziliensis. Revista da Sociedade Brasileira de Medicina Tropical 2006;39(1):22-6. - PubMed
Arevalo 2007
-
- Arevalo I, Tulliano G, Quispe A, Spaeth G, Matlashewski G, Llanos-Cuentas A, et al. Role of imiquimod and parenteral meglumine antimoniate in the initial treatment of cutaneous leishmaniasis. Clinical Infectious Diseases 2007;44(12):1549-54. - PubMed
Aronson 2016
-
- Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clinical Infectious Diseases 2016;63(12):e202-64. - PubMed
Badaro 2001
-
- Badaro R, Lobo I, Nakatani M, Muinos A, Netto EM, Coler RN, et al. Successful use of a defined antigen/GM-CSF adjuvant vaccine to treat mucosal Leishmaniasis refractory to antimony: a case report. Brazilian Journal of Infectious Diseases 2001;5(4):223-32. - PubMed
Barral 1995
-
- Barral A, Costa JM, Bittencourt AL, Barral-Netto M, Carvalho EM. Polar and subpolar diffuse cutaneous leishmaniasis in Brazil: clinical and immunopathologic aspects. International Journal of Dermatolology 1995;34(7):474-9. - PubMed
Baryza 1995
-
- Baryza MJ, Baryza GA. The Vancouver Scar Scale: an administration tool and its interrater reliability. Journal of Burn Care & Rehabilitation 1995;16(5):535-38. - PubMed
Beaumier 2013
-
- Beaumier CM, Gillespie PM, Hotez PJ, Bottazzi ME. New vaccines for neglected parasitic diseases and dengue. Translational Research 2013;162(3):144-55. - PubMed
Becker 1999
-
- Becker I, Volkow P, Velasco-Castrejon O, Salaiza-Suazo N, Berzunza-Cruz M, Dominguez JS, et al. The efficacy of pentamidine combined with allopurinol and immunotherapy for the treatment of patients with diffuse cutaneous leishmaniasis. Parasitology Research 1999;85(3):165-70. - PubMed
Berman 1981
-
- Berman JD, Neva FA. Effect of temperature on multiplication of Leishmania amastigotes within monocyte-derived macrophages in vitro. American Journal of Tropical Medicine and Hygiene 1981;30:318-21. - PubMed
Bern 2008
Beyrer 2007
-
- Beyrer C, Villar JC, Suwanvanichkij V, Singh S, Baral SD, Mills EJ. Neglected diseases, civil conflicts, and the right to health. Lancet 2007;370(9587):619-27. - PubMed
Biagi 1953
-
- Biagi F. Synthesis of 70 case histories of cutaneous leishmaniasis of Mexico (ulcer of the chicle gatherers). Medicina (Mex) 1953;33(682):385-96. - PubMed
Bigby 2003
-
- Bigby M, Williams H. Appraising systematic reviews and meta-analyses. Archives of Dermatology 2003;139(6):795-8. - PubMed
Boggild 2008
-
- Boggild AK, Miranda-Verastegui C, Espinosa D, Arevalo J, Martinez-Medina D, Llanos-Cuentas A, et al. Optimization of microculture and evaluation of miniculture for the isolation of Leishmania parasites from cutaneous lesions in Peru. American Journal of Tropical Medicine and Hygiene 2008;79(6):847-52. - PubMed
Boggild 2010
-
- Boggild AK, Ramos AP, Espinosa D, Valencia BM, Veland N, Miranda-Verastegui C, et al. Clinical and demographic stratification of test performance: a pooled analysis of five laboratory diagnostic methods for American cutaneous leishmaniasis. American Journal of Tropical Medicine and Hygiene 2010;83(2):345-50. - PMC - PubMed
Bomfim 2007
-
- Bomfim G, Andrade BB, Santos S, Clarencio J, Barral-Netto M, Barral A. Cellular analysis of cutaneous leishmaniasis lymphadenopathy: insights into the early phases of human disease. American Journal of Tropical Medicine and Hygiene 2007;77(5):854-9. - PubMed
Brito 2017b
Buates 1999
-
- Buates S, Matlashewski G. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. Journal of Infectious Diseases 1999;179(6):1485-94. - PubMed
Cardona‐Arias 2015
Carvalho 2015
Consigli 2006
-
- Consigli J, Danielo C, Gallerano V, Papa M, Guidi A. Cutaneous leishmaniasis: successful treatment with itraconazole. International Journal of Dermatolology 2006;45(1):46-9. - PubMed
Costa 1986
-
- Costa JM, Marsden PD, Llanos-Cuentas EA, Netto EM, Carvalho EM, Barral A, et al. Disseminated cutaneous leishmaniasis in a field clinic in Bahia, Brazil: a report of eight cases. Journal of Tropical and Medicine Hygiene 1986;89(6):319-23. - PubMed
Cota 2016
Covidence [Computer program]
-
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, accessed prior to 9 April 2019. Available at covidence.org.
Cummings 1997
-
- Cummings AM, Hedge JL, Laskey J. Ketoconazole impairs early pregnancy and the decidual cell response via alterations in ovarian function. Fundamental and Applied Toxicology 1997;40(2):238-46. - PubMed
Cupolillo 1994
-
- Cupolillo E, Grimaldi G Jr, Momen H. A general classification of New World Leishmania using numerical zymotaxonomy. American Journal of Tropical Medicine and Hygiene 1994;50(3):296-311. - PubMed
Cupolillo 2001
-
- Cupolillo E, Aguiar Alves F, Brahim LR, Naiff MF, Pereira LO, Oliveira-Neto MP, et al. Recent advances in the taxonomy of the New World leishmanial parasites. Medical Microbiology and Immunology 2001;190(1-2):57-60. - PubMed
Dantas 2014
Davies 1997
-
- Davies CR, Llanos-Cuentas EA, Campos P, Monge J, Villaseca P, Dye C. Cutaneous leishmaniasis in the Peruvian Andes: risk factors identified from a village cohort study. American Journal of Tropical Medicine and Hygiene 1997;56(1):85-95. - PubMed
Davies 2000
-
- Davies CR, Reithinger R, Campbell-Lendrum D, Feliciangeli D, Borges R, Rodriguez N. The epidemiology and control of leishmaniasis in Andean countries. Cadernos de Saude Publica / Ministerio da Saude, Fundacao Oswaldo Cruz, Escola Nacional de Saude Publica 2000;16(4):925-50. - PubMed
De Lima 2009
-
- De Lima H, Rodriguez N, Feliciangeli MD, Barrios MA, Sosa A, Agrela I, et al. Cutaneous leishmaniasis due to Leishmania chagasi/Le. infantum in an endemic area of Guarico State, Venezuela. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(7):721-6. - PubMed
De Macedo‐Silva 2013
Deeks 2019
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
Duthie 2012
Edwards 2000
-
- Edwards L. Imiquimod in clinical practice. Journal of the American Academy of Dermatology 2000;43(1 Pt 2):S12-7. - PubMed
Egger 1997
Enk 2015
-
- Enk CD, Nasereddin A, Alper R, Dan-Goor M, Jaffe CL, Wulf HC. Cutaneous leishmaniasis responds to daylight-activated photodynamic therapy: proof of concept for a novel self-administered therapeutic modality. British Journal of Dermatology 2015;172(5):1364-70. - PubMed
Evangelou 2011
-
- Evangelou G, Krasagakis K, Giannikaki E, Kruger-Krasagakis S, Tosca A. Successful treatment of cutaneous leishmaniasis with intralesional aminolevulinic acid photodynamic therapy. Photodermatology, Photoimmunology and Photomedicine 2011;27(5):254-6. - PubMed
FDA 2011
-
- Food And Drug Administration Drug Safety Communication. Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. www.fda.gov/Drugs/DrugSafety/ucm266030.htm (accessed prior to 23 January 2019).
FDA 2013
-
- Food And Drug Administration Drug Safety Communication. Nizoral (ketoconazole) oral tablets: Potentially fatal liver injury, risk of drug interactions and adrenal gland problems. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communica... (accessed 09 August 2020). - PubMed
FDA 2014
-
- Food And Drug Administration Drug Safety Communication. Sporanox (itraconazole) Oral Solution - Detailed view: Safety labeling changes approved by FDA Center for Drug Evaluation and Research (CDER). www.accessdata.fda.gov/drugsatfda_docs/label/2012/020657s027lbl.pdf (accessed prior to 23 January 2019).
FDA 2016
-
- Food And Drug Administration Drug Safety Communication. FDA evaluating study examining use of oral fluconazole (Diflucan) in pregnancy. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communica... (accessed prior to 23 January 2019).
Fernandez 2014
Fontenele e Silva 2013
-
- Fontenele e Silva JS, Galvao TF, Pereira MG, Silva MT. Treatment of American tegumentary leishmaniasis in special populations: a summary of evidence. Revista da Sociedade Brasileira de Medicina Tropical 2013;46(6):669-77. - PubMed
Fraga 2012
-
- Fraga J, Veland N, Montalvo AM, Praet N, Boggild AK, Valencia BM, et al. Accurate and rapid species typing from cutaneous and mucocutaneous leishmaniasis lesions of the New World. Diagnostic Microbiology and Infectious Disease 2012;74(2):142-50. - PubMed
Gallis 1990
-
- Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Revews of Infectious Diseases 1990;12(2):308-29. - PubMed
Galvão 2017
Galvão 2018
Galvão 2019
Gomes 2015
González 2010
-
- González U, Pinart M, Reveiz L, Rengifo-Pardo M, Tweed J, Macaya A, et al. Designing and reporting clinical trials on treatments for cutaneous leishmaniasis. Clinical Infectious Diseases 2010;51(4):409-19. - PubMed
González 2015
Graca 2012
-
- Graca GC, Volpini AC, Romero GA, Oliveira Neto MP, Hueb M, Porrozzi R, et al. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identification of the parasite species. Memórias do Instituto Oswaldo Cruz 2012;107(5):664-74. - PubMed
Guyatt 2008
Handler 2015
-
- Handler MZ, Patel PA, Kapila R, Al-Qubati Y, Schwartz RA. Cutaneous and mucocutaneous leishmaniasis: clinical perspectives. Journal of the American Academy of Dermatology 2015;73(6):897-908; quiz 909-10. - PubMed
Hashiguchi 2016
Heras‐Mosteiro 2017
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoeltzenbein 2013
Jin 2014
Karimkhani 2016
-
- Karimkhani C, Wanga V, Coffeng LE, Naghavi P, Dellavalle RP, Naghavi M. Global burden of cutaneous leishmaniasis: a cross-sectional analysis from the Global Burden of Disease Study 2013. Lancet Infectious Diseases 2016;16(5):584-91. - PubMed
Kim 2009
Lainson 1994
-
- Lainson R, Shaw JJ, Silveira FT, De Souza AA, Braga RR, Ishikawa EA. The dermal leishmaniases of Brazil, with special reference to the eco-epidemiology of the disease in Amazonia. Memórias do Instituto Oswaldo Cruz 1994;89(3):435-43. - PubMed
Lee 2002
-
- Lee N, Bertholet S, Debrabant A, Muller J, Duncan R, Nakhasi HL. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differentiation 2002;9(1):53-64. - PubMed
Lessa 2012
Llanos‐Cuentas 2008
-
- Llanos-Cuentas A, Tulliano G, Araujo-Castillo R, Miranda-Verastegui C, Santamaria-Castrellon G, Ramirez L, et al. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clinical Infectious Diseases 2008;46(2):223-31. - PubMed
Lopez 2012
López‐Carvajal 2016
Machado 2015
-
- Machado PR, Rosa ME, Guimaraes LH, Prates FV, Queiroz A, Schriefer A, et al. Treatment of disseminated leishmaniasis with liposomal amphotericin B. Clinical Infectious Diseases 2015;61(6):945-9. - PubMed
Mansueto 2014
-
- Mansueto P, Seidita A, Vitale G, Cascio A. Leishmaniasis in travelers: a literature review. Travel Medicine and Infectious Disease 2014;12(6 Pt A):563-81. - PubMed
Marsden 1979
-
- Marsden PD. Current concepts in parasitology. Leishmaniasis. New England Journal of Medicine 1979;300(7):350-2. - PubMed
Marsden 1994
-
- Marsden PD. Mucosal leishmaniasis due to Leishmania (Viannia) braziliensis L(V)b in Tres Bracos, Bahia-Brazil. Revista da Sociedade Brasileira de Medicina Tropical 1994;27(2):93-101. - PubMed
Marsden 1998
-
- Marsden PD, Lessa HA, Oliveira MR, Romero GA, Marotti JG, Sampaio RN, et al. Clinical observations of unresponsive mucosal leishmaniasis. American Journal of Tropical Medicine and Hygiene 1998;59(4):543-5. - PubMed
Mishra 2007
-
- Mishra J, Saxena A, Singh S. Chemotherapy of leishmaniasis: past, present and future. Current Medicinal Chemistry 2007;14(10):1153-69. - PubMed
Modabber 2007
Mondragon‐Shem 2015
Monteiro 2009
-
- Monteiro WM, Neitzke-Abreu HC, Ferreira ME, Melo GC, Barbosa Md, Lonardoni MV, et al. Population mobility and production of American tegumentary leishmaniasis in the State of Parana, southern Brazil. Revista da Sociedade Brasileira de Medicina Tropical 2009;42(5):509-14. - PubMed
Neto 2006
-
- Neto VL, Véras MM. American tegumentary leishmaniasis: fluconazole therapy. Revista Brasileira de Medicina de Família e Comunidade 2006;2(7):228-34.
Oliveira 2011
-
- Oliveira LF, Schubach AO, Martins MM, Passos SL, Oliveira RV, Marzochi MC, et al. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Tropica 2011;118(2):87-96. - PubMed
Oliveira 2015
Olliaro 2013
Olliaro 2018
Osorio 1998
-
- Osorio LE, Castillo CM, Ochoa MT. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: clinical characteristics. American Journal of Tropical Medicine and Hygiene 1998;59(1):49-52. - PubMed
Page 2019
-
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
PAHO 2015
-
- Pan American Health Organization. Leishmaniasis in the Americas: Treatment recommendations [Leishmaniasis en las Américas. recomendaciones para el tratamiento]. www.paho.org/hq/index.php?option=com_topics&view=rdmore&cid=6123... (accessed prior to 23 January 2019). [ISBN: 978-92-75-11752-1]
PAHO 2018
-
- Pan American Health Organization. Leishmaniasis in the Americas: Treatment recommendations. www.paho.org/hq/index.php?option=com_topics&view=rdmore&cid=6123... (accessed prior to 14 July 2020).
PAHO 2019a
-
- Pan American Health Organization. Leishmaniasis: Epidemiological Report in the Americas. iris.paho.org/xmlui/bitstream/handle/123456789/50505/Leishreport2019_eng... (accessed prior to 25 February 2020).
PAHO 2019b
-
- Pan American Health Organization. Manual of Procedures for Leishmaniases Surveillance and Control in the Americas. Washington, D.C: PAHO, 2019. [ISBN: 9789275120637]
Perez‐Florez 2016
Peterson 2003
-
- Peterson AT, Shaw J. Lutzomyia vectors for cutaneous leishmaniasis in Southern Brazil: ecological niche models, predicted geographic distributions, and climate change effects. International Journal of Parasitology 2003;33(9):919-31. - PubMed
Ponte‐Sucre 2003
Prestes 2015
-
- Prestes SR, Guerra JA, Romero GA, Magalhaes LK, Santana RA, Maciel MG, et al. Polymerase chain reaction-based method for the identification of Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in mucosal tissues conserved in paraffin. Revista da Sociedade Brasileira de Medicina Tropical 2015;48(5):555-9. - PubMed
Ramos 1996
-
- Ramos H, Valdivieso E, Gamargo M, Dagger F, Cohen BE. Amphotericin B kills unicellular leishmanias by forming aqueous pores permeable to small cations and anions. Journal of Membrane Biology 1996;152(1):65-75. - PubMed
Reed 2016
Reveiz 2013
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rojas 2002
-
- Rojas CA, Weigle KA, Barrera L, Collazos C. Surveillance and screening of American cutaneous leishmaniasis by Colombian primary health care workers using a clinical prediction rule. Transactions of the Royal Society of Tropical Medicine and Hygiene 2002;96(4):405-10. - PubMed
Romero 2001a
-
- Romero GA, Guerra MV, Paes MG, Macedo VO. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. American Journal of Tropical Medicine and Hygiene 2001;65(5):456-65. - PubMed
Romero 2001b
-
- Romero GA, Vinitius De Farias Guerra M, Gomes Paes M, De Oliveira Macedo V. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: clinical findings and diagnostic approach. Clinical Infectious Diseases 2001;32(9):1304-12. - PubMed
Romero 2005
-
- Romero GA, De la Gloria Orge M, De Farias Guerra MV, Paes MG, De Oliveira Macedo V, De Carvalho EM. Antibody response in patients with cutaneous leishmaniasis infected by Leishmania (Viannia) braziliensis or Leishmania (Viannia) guyanensis in Brazil. Acta Tropica 2005;93(1):49-56. - PubMed
Sacks 1983
-
- Sacks DL, Barral A, Neva F. Thermosensitivity patterns of Old vs. New World cutaneous strains of Leishmania growing within mouse peritoneal macrophages in vitro. American Journal of Tropical Medicine and Hygiene 1983;32(2):300-4. - PubMed
Salaiza‐Suazo 1999
-
- Salaiza-Suazo N, Volkow P, Tamayo R, Moll H, Gillitzer R, Perez-Torres A, et al. Treatment of two patients with diffuse cutaneous leishmaniasis caused by Leishmania mexicana modifies the immunohistological profile but not the disease outcome. Tropical Medicine and International Health 1999;4(12):801-11. - PubMed
Sands 1985
-
- Sands M, Kron MA, Brown RB. Pentamidine: a review. Reviews of Infectious Diseases 1985;7(5):625-34. - PubMed
Santrich 1990
-
- Santrich C, Segura I, Arias AL, Saravia NG. Mucosal disease caused by Leishmania braziliensis guyanensis. American Journal of Tropical Medicine and Hygiene 1990;42(1):51-5. - PubMed
Savioli 2006
Schonian 2010
-
- Schonian G, Mauricio I, Cupolillo E. Is it time to revise the nomenclature of Leishmania? Trends in Parasitology 2010;26(10):466-9. - PubMed
Sereno 2001
Sharifi 1998
-
- Sharifi I, FeKri AR, Aflatonian MR, Khamesipour A, Nadim A, Mousavi MR, et al. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet 1998;351(9115):1540-3. - PubMed
Shaw 1988
-
- Shaw JJ. Animal reservoirs of Leishmania in different ecological situations and their importance in the epidemiology of the disease. Memórias do Instituto Oswaldo Cruz 1988;83(Suppl 1):486-90. - PubMed
Sousa 2011
-
- Sousa AQ, Frutuoso MS, Moraes EA, Pearson RD, Pompeu MM. High-dose oral fluconazole therapy effective for cutaneous leishmaniasis due to Leishmania (Vianna) braziliensis. Clinical Infectious Diseases 2011;53(7):693-5. - PubMed
Sudhandiran 2003
-
- Sudhandiran G, Shaha C. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. Journal of Biological Chemistry 2003;278(27):25120-32. - PubMed
Sundar 2006
-
- Sundar S, Chatterjee M. Visceral leishmaniasis-current therapeutic modalities. Indian Journal of Medicine Research 2006;123(3):345-52. - PubMed
Sundar 2008
-
- Sundar S, Chakravarty J. Paromomycin in the treatment of leishmaniasis. Expert Opinion on Investigational Drugs 2008;17(5):787-94. - PubMed
Tojal da Silva 2006
-
- Tojal da Silva AC, Cupolillo E, Volpini AC, Almeida R, Romero GA. Species diversity causing human cutaneous leishmaniasis in Rio Branco, state of Acre, Brazil. Tropical Medicine & International Health 2006;11(9):1388-98. - PubMed
Toledo 2013
-
- Toledo AC Jr, Da Silva RE, Carmo RF, Amaral TA, Luz ZM, Rabello A. Assessment of the quality of life of patients with cutaneous leishmaniasis in Belo Horizonte, Brazil, 2009-2010. A pilot study. Transactions of the Royal Society of Tropical Medicine 2013;107(5):335-6. - PubMed
Tracy 2001
-
- Tracy JW, Webster LT Jr. Drugs used in the chemotherapy of protozoal infections. In: Hardman JG, Limbird LE, Gilman AG, editors(s). The Pharmacological Basis of Therapeutics. 10th edition. New York: McGraw-Hill, 2001.
Turetz 2002
-
- Turetz ML, Machado PR, Ko AI, Alves F, Bittencourt A, Almeida RP, et al. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. Journal of Infectious Diseases 2002;186(12):1829-34. - PubMed
Verma 2004
Weigle 1987
-
- Weigle KA, De Davalos M, Heredia P, Molineros R, Saravia NG, D'Alessandro A. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. American Journal of Tropical Medicine and Hygiene 1987;36(3):489-96. - PubMed
Weigle 1991
-
- Weigle KA, Valderrama L, Arias AL, Santrich C, Saravia NG. Leishmanin skin test standardization and evaluation of safety, dose, storage, longevity of reaction and sensitization. American Journal of Tropical Medicine and Hygiene 1991;44(3):260-71. - PubMed
Weigle 1993
-
- Weigle KA, Escobar M, Arias AL, Martinez F, Rojas C. A clinical prediction rule for American cutaneous leishmaniasis in Colombia. International Journal of Epidemiology 1993;22(3):548-58. - PubMed
WHO 2010
-
- World Health Organization. Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22-26 March 2010. apps.who.int/iris/handle/10665/44412 (accessed prior to 23 January 2019).
Wortmann 2010
Yadon 2003
-
- Yadon ZE, Rodrigues LC, Davies CR, Quigley MA. Indoor and peridomestic transmission of American cutaneous leishmaniasis in northwestern Argentina: a retrospective case-control study. American Journal of Tropical Medicine and Hygiene 2003;68(5):519-26. - PubMed
Yang 2012
-
- Yang YG, Zou XB, Zhao H, Zhang YJ, Li HJ. Photodynamic therapy of condyloma acuminata in pregnant women. Chinese Medical Journal (Engl) 2012;125(16):2925-8. - PubMed
Zajtchuk 1989
-
- Zajtchuk JT, Casler JD, Netto EM, Grogl M, Neafie RC, Hessel CR, et al. Mucosal leishmaniasis in Brazil. Laryngoscope 1989;99(9):925-39. - PubMed
References to other published versions of this review
González 2004
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

