Mechanism of traumatic heterotopic ossification: In search of injury-induced osteogenic factors
- PMID: 32853465
- PMCID: PMC7576286
- DOI: 10.1111/jcmm.15735
Mechanism of traumatic heterotopic ossification: In search of injury-induced osteogenic factors
Abstract
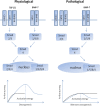
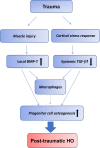
Heterotopic ossification (HO) is a pathological condition of abnormal bone formation in soft tissue. Three factors have been proposed as required to induce HO: (a) osteogenic precursor cells, (b) osteoinductive agents and (c) an osteoconductive environment. Since Urist's landmark discovery of bone induction in skeletal muscle tissue by demineralized bone matrix, it is generally believed that skeletal muscle itself is a conductive environment for osteogenesis and that resident progenitor cells in skeletal muscle are capable of differentiating into osteoblast to form bone. However, little is known about the naturally occurring osteoinductive agents that triggered this osteogenic response in the first place. This article provides a review of the emerging findings regarding distinct types of HO to summarize the current understanding of HO mechanisms, with special attention to the osteogenic factors that are induced following injury. Specifically, we hypothesize that muscle injury-induced up-regulation of local bone morphogenetic protein-7 (BMP-7) level, combined with glucocorticoid excess-induced down-regulation of circulating transforming growth factor-β1 (TGF-β1) level, could be an important causative mechanism of traumatic HO formation.
Keywords: bone morphogenetic protein; dystrophic calcification; glucocorticoid; heterotopic ossification; muscle injury; transforming growth factor-β1.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures


References
-
- Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43:346‐353. - PubMed
-
- Eisenstein N, Stapley S, Grover L. Post‐traumatic heterotopic ossification: an old problem in need of new solutions. J Orthop Res. 2018;36:1061‐1068. - PubMed
-
- Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525‐527. - PubMed
-
- Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37:129‐136. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

