The Ubiquitin System and A20: Implications in Health and Disease
- PMID: 32853526
- PMCID: PMC7755949
- DOI: 10.1177/0022034520949486
The Ubiquitin System and A20: Implications in Health and Disease
Abstract
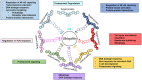
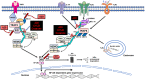
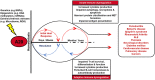
Inflammation is triggered by stimulation of innate sensors that recognize pathogens, chemical and physical irritants, and damaged cells subsequently initiating a well-orchestrated adaptive immune response. Immune cell activation is a strictly regulated and self-resolving process supported by an array of negative feedback mechanisms to sustain tissue homeostasis. The disruption of these regulatory pathways forms the basis of chronic inflammatory diseases, including periodontitis. Ubiquitination, a covalent posttranslational modification of target proteins with ubiquitin, has a profound effect on the stability and activity of its substrates, thereby regulating the immune system at molecular and cellular levels. Through the cooperative actions of E3 ubiquitin ligases and deubiquitinases, ubiquitin modifications are implicated in several biological processes, including proteasomal degradation, transcriptional regulation, regulation of protein-protein interactions, endocytosis, autophagy, DNA repair, and cell cycle regulation. A20 (tumor necrosis factor α-induced protein 3 or TNFAIP3) is a ubiquitin-editing enzyme that mainly functions as an endogenous regulator of inflammation through termination of nuclear factor (NF)-κB activation as part of a negative feedback loop. A20 interacts with substrates that reside downstream of immune sensors, including Toll-like receptors, nucleotide-binding oligomerization domain-containing receptors, lymphocyte receptors, and cytokine receptors. Due to its pleiotropic functions as a ubiquitin binding protein, deubiquitinase and ubiquitin ligase, and its versatile role in various signaling pathways, aberrant A20 levels are associated with numerous conditions such as rheumatoid arthritis, diabetes, systemic lupus erythematosus, inflammatory bowel disease, psoriasis, Sjögren syndrome, coronary artery disease, multiple sclerosis, cystic fibrosis, asthma, cancer, neurological disorders, and aging-related sequelae. Similarly, A20 has recently been implicated as an essential regulator of inflammation in the oral cavity. This review presents information on the ubiquitin system and regulation of NF-κB by ubiquitination using A20 as a representative molecule and highlights how the dysregulation of this system can lead to several immune pathologies, including oral cavity-related disorders mainly focusing on periodontitis.
Keywords: NF-κB; TLR; cytokines; inflammation; periodontitis; ubiquitination.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures

Similar articles
-
Expression, biological activities and mechanisms of action of A20 (TNFAIP3).Biochem Pharmacol. 2010 Dec 15;80(12):2009-20. doi: 10.1016/j.bcp.2010.06.044. Epub 2010 Jul 3. Biochem Pharmacol. 2010. PMID: 20599425 Review.
-
De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling.Nature. 2004 Aug 5;430(7000):694-9. doi: 10.1038/nature02794. Epub 2004 Jul 18. Nature. 2004. PMID: 15258597
-
Increased A20-E3 ubiquitin ligase interactions in bid-deficient glia attenuate TLR3- and TLR4-induced inflammation.J Neuroinflammation. 2018 May 2;15(1):130. doi: 10.1186/s12974-018-1143-3. J Neuroinflammation. 2018. PMID: 29720226 Free PMC article.
-
Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20.J Biol Chem. 2006 Jan 27;281(4):2095-103. doi: 10.1074/jbc.M510326200. Epub 2005 Nov 23. J Biol Chem. 2006. PMID: 16306043
-
The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology.Trends Immunol. 2009 Aug;30(8):383-91. doi: 10.1016/j.it.2009.05.007. Epub 2009 Jul 28. Trends Immunol. 2009. PMID: 19643665 Review.
Cited by
-
The role of lipoprotein‑associated phospholipase A2 in inflammatory response and macrophage infiltration in sepsis and the regulatory mechanisms.Funct Integr Genomics. 2024 Sep 30;24(5):178. doi: 10.1007/s10142-024-01460-6. Funct Integr Genomics. 2024. PMID: 39343830
-
Delivering miR-23b-3p by small extracellular vesicles to promote cell senescence and aberrant lipid metabolism.BMC Biol. 2025 Feb 11;23(1):41. doi: 10.1186/s12915-025-02143-9. BMC Biol. 2025. PMID: 39934790 Free PMC article.
-
Activating UCHL1 through the CRISPR activation system promotes cartilage differentiation mediated by HIF-1α/SOX9.J Cell Mol Med. 2024 Sep;28(17):e70051. doi: 10.1111/jcmm.70051. J Cell Mol Med. 2024. PMID: 39223923 Free PMC article.
-
The effect of the "Oral-Gut" axis on periodontitis in inflammatory bowel disease: A review of microbe and immune mechanism associations.Front Cell Infect Microbiol. 2023 Feb 27;13:1132420. doi: 10.3389/fcimb.2023.1132420. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36923589 Free PMC article. Review.
-
Gastric cancer secreted miR-214-3p inhibits the anti-angiogenesis effect of apatinib by suppressing ferroptosis in vascular endothelial cells.Oncol Res. 2024 Feb 6;32(3):489-502. doi: 10.32604/or.2023.046676. eCollection 2024. Oncol Res. 2024. PMID: 38370339 Free PMC article.
References
-
- Aeschlimann FA, Batu ED, Canna SW, Go E, Gül A, Hoffmann P, Leavis HL, Ozen S, Schwartz DM, Stone DL, et al. 2018. A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-κB-mediated autoinflammatory disease. Ann Rheum Dis. 77(5):728–735. - PubMed
-
- Bartold PM, Van Dyke TE. 2017. Host modulation: controlling the inflammation to control the infection. Periodontol 2000. 75(1):317–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

