Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial
- PMID: 32853559
- PMCID: PMC7493715
- DOI: 10.1016/S0140-6736(20)31788-8
Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial
Abstract
Background: The anti-progesterone drug mifepristone and the prostaglandin misoprostol can be used to treat missed miscarriage. However, it is unclear whether a combination of mifepristone and misoprostol is more effective than administering misoprostol alone. We investigated whether treatment with mifepristone plus misoprostol would result in a higher rate of completion of missed miscarriage compared with misoprostol alone.
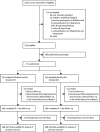
Methods: MifeMiso was a multicentre, double-blind, placebo-controlled, randomised trial in 28 UK hospitals. Women were eligible for enrolment if they were aged 16 years and older, diagnosed with a missed miscarriage by pelvic ultrasound scan in the first 14 weeks of pregnancy, chose to have medical management of miscarriage, and were willing and able to give informed consent. Participants were randomly assigned (1:1) to a single dose of oral mifepristone 200 mg or an oral placebo tablet, both followed by a single dose of vaginal, oral, or sublingual misoprostol 800 μg 2 days later. Randomisation was managed via a secure web-based randomisation program, with minimisation to balance study group assignments according to maternal age (<30 years vs ≥30 years), body-mass index (<35 kg/m2vs ≥35 kg/m2), previous parity (nulliparous women vs parous women), gestational age (<70 days vs ≥70 days), amount of bleeding (Pictorial Blood Assessment Chart score; ≤2 vs ≥3), and randomising centre. Participants, clinicians, pharmacists, trial nurses, and midwives were masked to study group assignment throughout the trial. The primary outcome was failure to spontaneously pass the gestational sac within 7 days after random assignment. Primary analyses were done according to intention-to-treat principles. The trial is registered with the ISRCTN registry, ISRCTN17405024.
Findings: Between Oct 3, 2017, and July 22, 2019, 2595 women were identified as being eligible for the MifeMiso trial. 711 women were randomly assigned to receive either mifepristone and misoprostol (357 women) or placebo and misoprostol (354 women). 696 (98%) of 711 women had available data for the primary outcome. 59 (17%) of 348 women in the mifepristone plus misoprostol group did not pass the gestational sac spontaneously within 7 days versus 82 (24%) of 348 women in the placebo plus misoprostol group (risk ratio [RR] 0·73, 95% CI 0·54-0·99; p=0·043). 62 (17%) of 355 women in the mifepristone plus misoprostol group required surgical intervention to complete the miscarriage versus 87 (25%) of 353 women in the placebo plus misoprostol group (0·71, 0·53-0·95; p=0·021). We found no difference in incidence of adverse events between the study groups.
Interpretation: Treatment with mifepristone plus misoprostol was more effective than misoprostol alone in the management of missed miscarriage. Women with missed miscarriage should be offered mifepristone pretreatment before misoprostol to increase the chance of successful miscarriage management, while reducing the need for miscarriage surgery.
Funding: UK National Institute for Health Research Health Technology Assessment Programme.
This is an Open Access article under the CC BY-NC-ND 4.0 license.
Figures
Comment in
-
Medical management of miscarriage with mifepristone.Lancet. 2020 Sep 12;396(10253):737-739. doi: 10.1016/S0140-6736(20)31789-X. Epub 2020 Aug 24. Lancet. 2020. PMID: 32853558 No abstract available.
-
Excerpts from the World Medical Literature: Obstetrics.J Obstet Gynaecol Can. 2023 Jul;45(7):479-481. doi: 10.1016/j.jogc.2023.04.018. J Obstet Gynaecol Can. 2023. PMID: 37400184 No abstract available.
References
-
- Cantwell R, Clutton-Brock T, Cooper G. Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG. 2011;118(suppl 1):1–203. - PubMed
-
- Farren J, Jalmbrant M, Falconieri N. Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: a multicenter, prospective, cohort study. Am J Obstet Gynecol. 2020;222:367. - PubMed
-
- National Institute for Health and Clinical Excellence Ectopic pregnancy and miscarriage: diagnosis and initial management. April 17, 2019. https://www.nice.org.uk/guidance/ng126 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous