Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: phase 1 results of an open-label, multicentre, phase 1/2 trial
- PMID: 32853585
- PMCID: PMC7737486
- DOI: 10.1016/S2352-3026(20)30221-0
Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: phase 1 results of an open-label, multicentre, phase 1/2 trial
Abstract
Background: Recognising that the immune suppressive microenvironment promotes tumour growth in Hodgkin lymphoma, we hypothesised that activating immunity might augment the activity of targeted chemotherapy. We evaluated the safety and activity of combinations of brentuximab vedotin with nivolumab or ipilimumab, or both in patients with relapsed or refractory Hodgkin lymphoma.
Methods: In this multicentre, open-label, phase 1/2 trial, patients with relapsed or refractory Hodgkin lymphoma aged 18 years or older who had relapsed after at least one line of therapy, with an Eastern Cooperative Oncology Group performance status of 2 or lower, and adequate organ and marrow function, with no pulmonary dysfunction were eligible for inclusion. Phase 1 primary objectives were to determine the maximum tolerated dose and dose limiting toxicities of brentuximab vedotin combined with ipilimumab (ipilimumab group), nivolumab (nivolumab group), or both (triplet therapy group) using a 3 + 3 dose escalation design with expansion cohorts. During the dose escalation phase, patients were enrolled sequentially into one of six cohorts: in the ipilimumab group fixed brentuximab vedotin 1·8 mg/kg with ipilimumab 1 mg/kg (cohort A) or 3 mg/kg (cohort B); in the nivolumab group fixed nivolumab 3 mg/kg with brentuximab vedotin 1·2 mg/kg (cohort D) or 1·8 mg/kg (cohort E); and in the triplet therapy group fixed nivolumab 3 mg/kg and ipilimumab 1 mg/kg with brentuximab vedotin 1·2 mg/kg (cohort G) or 1·8 mg/kg (cohort H). Additional patients were enrolled in the expansion phase at the same doses of cohorts B, E, and H. All drugs were given intravenously; brentuximab vedotin and nivolumab were given every 3 weeks, ipilimumab was given every 6 weeks in the ipilimumab group and every 12 weeks in the triplet therapy group. All eligible and treated patients were included in the analysis. This phase 1/2 study is registered with ClinicalTrials.gov, NCT01896999. The phase 2, randomised portion of the trial is still enrolling.
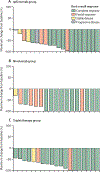
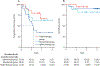
Findings: Between March 7, 2014, and Dec 28, 2017, 64 patients were enrolled; two patients in the ipilimumab group and one patient in the nivolumab group were excluded due to ineligibility after enrolment and 61 were evaluable. A total of six dose limiting toxicities were reported in four patients, and the doses used in cohorts B, E, and H were established as maximum tolerated doses and patients were subsequently enrolled onto expansion cohorts (C, F, and I) with these schedules. There were ten (43%) grade 3-4 treatment related adverse events in the ipilimumab group, three (16%) in the nivolumab group, and 11 (50%) in the triplet therapy group including: eight (13%) of 64 patients reporting rash, and colitis, gastritis, pancreatitis and arthritis, and diabetic ketoacidosis each occurring in one (2%) patient. There were two (3%) treatment related deaths, one in the nivolumab group and one in the triplet therapy group. The overall response rate was 76% (95% CI 53-92) in the ipilimumab group, 89% (65-99) in the nivolumab group, and 82% (60-95) in the triplet therapy group, and the complete response rate was 57% (95% CI 34-78%) in the ipilimumab group, 61% (36-83%) in the nivolumab group, and 73% (50-89%) in the triplet therapy group. With a median follow-up of 2·6 years (IQR 1·8-2·9) in the ipilimumab group, 2·4 years (2·2-2·6) in the nivolumab group, and 1·7 years (1·6-1·9) in the triplet therapy group, median progression-free survival is 1·2 years (95% CI 1·7-not reached) in the ipilimumab group, but was not reached in the other two treatment groups. Median overall survival has not been reached in any of the groups.
Interpretation: There are clear differences in activity and toxicity of the three combination regimens. The tolerability and preliminary activity for the two most active regimens, brentuximab vedotin with nivolumab and the triplet therapy, are being compared in a randomised phase 2 trial (NCT01896999).
Funding: Eastern Cooperative Oncology Group-American College of Radiology Imaging Network and the National Cancer Institute of the National Institutes of Health.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Total immunotherapy for Hodgkin lymphoma.Lancet Haematol. 2020 Sep;7(9):e629-e630. doi: 10.1016/S2352-3026(20)30220-9. Lancet Haematol. 2020. PMID: 32853579 No abstract available.
References
-
- Diefenbach CS, Sabado R, Clark-Garvey S, Cruz C, Vengco I, Rojas CN, et al. PD-1 Is Elevated on the Peripheral Blood T Cell Subsets of Patients with Relapsed/Refractory Hodgkin Lymphoma Compared to Normal Volunteers. ASH Annual Meeting Abstracts; November 18, 20112011. p. 4860.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

