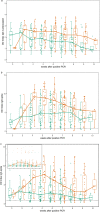
SARS-CoV-2 IgG and IgA antibody response is gender dependent; and IgG antibodies rapidly decline early on
- PMID: 32853597
- PMCID: PMC7445467
- DOI: 10.1016/j.jinf.2020.08.032
SARS-CoV-2 IgG and IgA antibody response is gender dependent; and IgG antibodies rapidly decline early on
Figures

Comment on
-
The role of serology for COVID-19 control: Population, kinetics and test performance do matter.J Infect. 2020 Aug;81(2):e91-e92. doi: 10.1016/j.jinf.2020.05.019. Epub 2020 May 15. J Infect. 2020. PMID: 32417311 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

