Safety and effectiveness of azithromycin in patients with COVID-19: An open-label randomised trial
- PMID: 32853672
- PMCID: PMC7445147
- DOI: 10.1016/j.ijantimicag.2020.106143
Safety and effectiveness of azithromycin in patients with COVID-19: An open-label randomised trial
Abstract
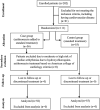
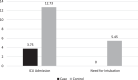
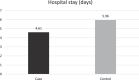
As no specific pharmacological treatment has been validated for use in coronavirus disease 2019 (COVID-19), we aimed to assess the effectiveness of azithromycin (AZM) in these patients at a referral centre in Iran. An open-label, randomised controlled trial was conducted on patients with laboratory-confirmed COVID-19. A total of 55 patients in the control group receiving hydroxychloroquine (HCQ) and lopinavir/ritonavir (LPV/r) were compared with 56 patients in the case group who in addition to the same regimen also received AZM. Patients with prior cardiac disease were excluded from the study. Furthermore, patients from the case group were assessed for cardiac arrythmia risk based on the American College of Cardiology (ACC) risk assessment for use of AZM and HCQ. The main outcome measures were vital signs, SpO2 levels, duration of hospitalisation, need for and length of intensive care unit admission, mortality rate and results of 30-day follow-up after discharge. Initially, there was no significant difference between the general conditions and vital signs of the two groups. The SpO2 levels at discharge were significantly higher, the respiratory rate was lower and the duration of admission was shorter in the case group. There was no significant difference in the mortality rate between the two groups. Patients who received AZM in addition to HCQ and LPV/r had a better general condition. HCQ+AZM combination may be beneficial for individuals who are known to have a very low underlying risk for cardiac arrhythmia based on the ACC criteria.
Keywords: Azithromycin; COVID-19; Hydroxychloroquine; Lopinavir; Ritonavir; SARS-CoV-2.
Copyright © 2020 Elsevier Ltd and International Society of Antimicrobial Chemotherapy. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

