Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: a nationwide observational study of 8075 participants
- PMID: 32853673
- PMCID: PMC7444610
- DOI: 10.1016/j.ijantimicag.2020.106144
Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: a nationwide observational study of 8075 participants
Abstract
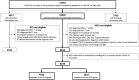
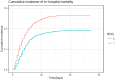
Hydroxychloroquine (HCQ) has been largely used and investigated as therapy for COVID-19 across various settings at a total dose usually ranging from 2400 mg to 9600 mg. In Belgium, off-label use of low-dose HCQ (total 2400 mg over 5 days) was recommended for hospitalised patients with COVID-19. We conducted a retrospective analysis of in-hospital mortality in the Belgian national COVID-19 hospital surveillance data. Patients treated either with HCQ monotherapy and supportive care (HCQ group) were compared with patients treated with supportive care only (no-HCQ group) using a competing risks proportional hazards regression with discharge alive as competing risk, adjusted for demographic and clinical features with robust standard errors. Of 8075 patients with complete discharge data on 24 May 2020 and diagnosed before 1 May 2020, 4542 received HCQ in monotherapy and 3533 were in the no-HCQ group. Death was reported in 804/4542 (17.7%) and 957/3533 (27.1%), respectively. In the multivariable analysis, mortality was lower in the HCQ group compared with the no-HCQ group [adjusted hazard ratio (aHR) = 0.684, 95% confidence interval (CI) 0.617-0.758]. Compared with the no-HCQ group, mortality in the HCQ group was reduced both in patients diagnosed ≤5 days (n = 3975) and >5 days (n = 3487) after symptom onset [aHR = 0.701 (95% CI 0.617-0.796) and aHR = 0.647 (95% CI 0.525-0.797), respectively]. Compared with supportive care only, low-dose HCQ monotherapy was independently associated with lower mortality in hospitalised patients with COVID-19 diagnosed and treated early or later after symptom onset.
Keywords: COVID-19; Hydroxychloroquine; Mortality; Observational study; SARS-CoV-2.
Copyright © 2020 Elsevier Ltd and International Society of Antimicrobial Chemotherapy. All rights reserved.
Figures

Comment in
-
Perceived efficacy of hydroxychloroquine in observational studies: Results of the confounding effect of "goals of care".Int J Antimicrob Agents. 2021 Apr;57(4):106308. doi: 10.1016/j.ijantimicag.2021.106308. Epub 2021 Feb 17. Int J Antimicrob Agents. 2021. PMID: 33609717 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

