Double-Blind Placebo-Controlled Randomized Clinical Trial of Neurofeedback for Attention-Deficit/Hyperactivity Disorder With 13-Month Follow-up
- PMID: 32853703
- PMCID: PMC7904968
- DOI: 10.1016/j.jaac.2020.07.906
Double-Blind Placebo-Controlled Randomized Clinical Trial of Neurofeedback for Attention-Deficit/Hyperactivity Disorder With 13-Month Follow-up
Abstract
Objective: To determine whether theta/beta-ratio (TBR) electroencephalographic biofeedback (neurofeedback [NF]) has a specific effect on attention-deficit/hyperactivity disorder (ADHD) beyond nonspecific benefit.
Method: In a 2-site double-blind randomized clinical trial, 144 children aged 7 to 10 years with rigorously diagnosed moderate/severe ADHD and theta/beta-ratio (TBR) ≥4.5 were randomized 3:2 to deliberate TBR downtraining versus a control of equal duration, intensity, and appearance. Two early dropouts left 142 children for modified intent-to-treat analysis. The control used prerecorded electroencephalograms with the participant's artifacts superimposed. Treatment was programmed via Internet by an off-site statistician-guided co-investigator. Fidelity was 98.7% by trainers/therapists and 93.2% by NF expert monitor. The primary outcome was parent- and teacher-rated inattention; analysis was mixed-effects regression. Because the expense and effort of NF can be justified only by enduring benefit, follow-ups were integrated.
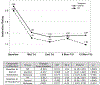
Results: Blinding was excellent. Although both groups showed significant improvement (p < .001, d = 1.5) in parent/teacher-rated inattention from baseline to treatment end and 13-month follow-up, NF was not significantly superior to the control condition at either time point on this primary outcome (d = 0.01, p = .965 at treatment end; d = 0.23, p = .412 at 13-month follow-up). Responders (Clinical Global Impression-Improvement [CGI-I] = 1-2) were 61% of NF and 54% of controls (p = .36). Adverse events were distributed proportionally between treatments. The 13-month follow-up found nonsignificant improvement from treatment end for NF (d = 0.1), with mild deterioration for controls (d = -0.07). NF required significantly less medication at follow-up (p = .012).
Conclusion: This study does not support a specific effect of deliberate TBR NF at either treatment end or 13-month follow-up. Participants will be reassessed at 25-month follow-up.
Clinical trial registration information: Double-Blind 2-Site Randomized Clinical Trial of Neurofeedback for ADHD; https://clinicaltrials.gov/; NCT02251743.
Keywords: ADHD; attention-deficit; clinical trials; double-blind; neurofeedback.
Copyright © 2020 American Academy of Child and Adolescent Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Editorial: The Complexity of Neurofeedback and Control of Placebo Effects.J Am Acad Child Adolesc Psychiatry. 2021 Jul;60(7):811-812. doi: 10.1016/j.jaac.2021.05.008. Epub 2021 May 25. J Am Acad Child Adolesc Psychiatry. 2021. PMID: 34048885
References
-
- Spetie L, and Arnold LE. Attention deficit hyperactivity disorder. In: : Martin A, and Volkmar R, editor Lewis’ child and adolescent psychiatry: A comprehensive textbook. 4th. Baltimore, MD: Wolters Kluwer/Lippincott Williams and Wilkins; 2007:430–453.
-
- Swanson JM, Kraemer HC, Hinshaw SP, Arnold LE, Conners CK, Abikoff HB, Clevenger W, Davies M, Elliott GR, Greenhill LL, Hechtman L, Hoza B, Jensen PS, March JS, Newcorn JH, Owens EB, Pelham WE, Schiller E, Severe JB, Simpson S, Vitiello B, Wells K, Wigal T, and Wu M. Clinical relevance of the primary findings of the MTA: success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40(2):168–79. doi:10.1097/00004583-200102000-00011. - DOI - PubMed
-
- Arns M, Vollebregt M, Palmer D, Spooner C, Gordon E, Kohn M, Clarke S, Elliott G, and Buitelaar J. Electroencephalographic biomarkers as predictors of methylphenidate response in attention-deficit/hyperactivity disorder. European Neuropsychopharmacology. 2018. doi:10.1016/j.euroneuro.2018.06.002. - DOI - PubMed
-
- Jensen PS, Arnold LE, Swanson JM, Vitiello B, Abikoff HB, Greenhill LL, Hechtman L, Hinshaw SP, Pelham WE, Wells KC, Conners CK, Elliott GR, Epstein JN, Hoza B, March JS, Molina BSG, Newcorn JH, Severe JB, Wigal T, Gibbons RD, and Hur K. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989–1002. doi:10.1097/CHI.0b013e3180686d48. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

