Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis
- PMID: 32853978
- PMCID: PMC7420982
- DOI: 10.1016/j.thromres.2020.08.020
Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis
Abstract
Background: Venous thromboembolism (VTE) may complicate the course of Coronavirus Disease 2019 (COVID-19).
Objectives: To evaluate the incidence of VTE in patients with COVID-19.
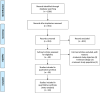
Methods: MEDLINE, EMBASE, and PubMed were searched up to 24th June 2020 for studies that evaluated the incidence of VTE, including pulmonary embolism (PE) and/or deep vein thrombosis (DVT), in patients with COVID-19. Pooled proportions with corresponding 95% confidence intervals (CI) and prediction intervals (PI) were calculated by random-effect meta-analysis.
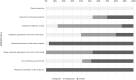
Results: 3487 patients from 30 studies were included. Based on very low-quality evidence due to heterogeneity and risk of bias, the incidence of VTE was 26% (95% PI, 6%-66%). PE with or without DVT occurred in 12% of patients (95% PI, 2%-46%) and DVT alone in 14% (95% PI, 1%-75%). Studies using standard algorithms for clinically suspected VTE reported PE in 13% of patients (95% PI, 2%-57%) and DVT in 6% (95% PI, 0%-60%), compared to 11% (95% PI, 2%-46%) and 24% (95% PI, 2%-85%) in studies using other diagnostic strategies or patient sampling. In patients admitted to intensive care units, VTE occurred in 24% (95% PI, 5%-66%), PE in 19% (95% PI, 6%-47%), and DVT alone in 7% (95% PI, 0%-69%). Corresponding values in general wards were respectively 9% (95% PI, 0%-94%), 4% (95% PI, 0%-100%), and 7% (95% CI, 1%-49%).
Conclusions: VTE represents a frequent complication in hospitalized COVID-19 patients and often occurs as PE. The threshold for clinical suspicion should be low to trigger prompt diagnostic testing.
Keywords: Anticoagulants; COVID-19; Pulmonary embolism; SARS virus; Venous thromboembolism.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
E. Valeriani, E. Porreca, A. Rutjes has nothing to disclose; A. Porfidia received personal fees and non-financial support from Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Aspen, Novartis outside the submitted work; R. Pola received personal fees and non-financial support from Boehringer Ingelheim, Bayer, Daiichi Sankyo, Pfizer, Aspen, Novartis outside the submitted work; M. Di Nisio received personal fees from Bayer, Daiichi Sankyo, Pfizer, Leo Pharma, and Sanofi outside the submitted work.
Figures



Comment in
-
Review of article: Porfidia, A., Valeriani, E., Pola, R., Porreca, E., Rutjes, A.W.S., Di Nisio, M. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thrombosis Research. 2020;196:67-74.J Vasc Nurs. 2020 Dec;38(4):193-194. doi: 10.1016/j.jvn.2020.11.002. J Vasc Nurs. 2020. PMID: 33279110 Free PMC article. No abstract available.
References
-
- World Health Organization Coronavirus disease 2019 (COVID-19) situation report – 101. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for Covid Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. - DOI - PMC - PubMed
-
- Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., Brown T.S., Nigoghossian C., Zidar D.A., Haythe J., Brodie D., Beckman J.A., Kirtane A.J., Stone G.W., Krumholz H.M., Parikh S.A. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.03.031. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

