The neuropeptide calcitonin gene-related peptide alpha is essential for bone healing
- PMID: 32853990
- PMCID: PMC7452713
- DOI: 10.1016/j.ebiom.2020.102970
The neuropeptide calcitonin gene-related peptide alpha is essential for bone healing
Abstract
Background: Impaired fracture healing represents an ongoing clinical challenge, as treatment options remain limited. Calcitonin gene-related peptide (CGRP), a neuropeptide targeted by emerging anti-migraine drugs, is also expressed in sensory nerve fibres innervating bone tissue.
Method: Bone healing following a femoral osteotomy stabilized with an external fixator was analysed over 21 days in αCGRP-deficient and WT mice. Bone regeneration was evaluated by serum analysis, µCT analysis, histomorphometry and genome-wide expression analysis. Bone-marrow-derived osteoblasts and osteoclasts, as well as the CGRP antagonist olcegepant were employed for mechanistic studies.
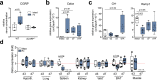
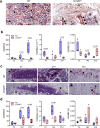
Findings: WT mice with a femoral fracture display increased CGRP serum levels. αCGRP mRNA expression after skeletal injury is exclusively induced in callus tissue, but not in other organs. On protein level, CGRP and its receptor, calcitonin receptor-like receptor (CRLR) complexing with RAMP1, are differentially expressed in the callus during bone regeneration. On the other hand, αCGRP-deficient mice display profoundly impaired bone regeneration characterised by a striking reduction in the number of bone-forming osteoblasts and a high rate of incomplete callus bridging and non-union. As assessed by genome-wide expression analysis, CGRP induces the expression of specific genes linked to ossification, bone remodeling and adipogenesis. This suggests that CGRP receptor-dependent PPARγ signaling plays a central role in fracture healing.
Interpretation: This study demonstrates an essential role of αCGRP in orchestrating callus formation and identifies CGRP receptor agonism as a potential approach to stimulate bone regeneration. Moreover, as novel agents blocking CGRP or its receptor CRLR are currently introduced clinically for the treatment of migraine disorders, their potential negative impact on bone regeneration warrants clinical investigation.
Funding: This work was funded by grants from the Else-Kröner-Fresenius-Stiftung (EKFS), the Deutsche Forschungsgemeinschaft (DFG), and the Berlin Institute of Health (BIH).
Keywords: Bone regeneration; CRLR; Fracture; Neuropeptides; Olcegepant; Osteoblasts; Osteoclasts; αCGRP.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declarations of Competing Interest All authors state that they have no conflict of interest.
Figures






References
-
- Tzioupis C., Giannoudis P.V. Prevalence of long-bone non-unions. Injury. 2007;38(Suppl 2):S3–S9. - PubMed
-
- Hak D.J., Fitzpatrick D., Bishop J.A., Marsh J.L., Tilp S., Schnettler R. Delayed union and non-unions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45(Suppl 2):S3–S7. - PubMed
-
- Hankenson K.D., Gagne K., Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv Drug Deliv Rev. 2015;94:3–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

