Back to the Future: Rethinking the Great Potential of lncRNAS for Optimizing Chemotherapeutic Response in Ovarian Cancer
- PMID: 32854207
- PMCID: PMC7564391
- DOI: 10.3390/cancers12092406
Back to the Future: Rethinking the Great Potential of lncRNAS for Optimizing Chemotherapeutic Response in Ovarian Cancer
Abstract
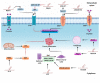
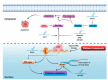
Ovarian cancer (OC) is one of the most fatal cancers in women worldwide. Currently, platinum- and taxane-based chemotherapy is the mainstay for the treatment of OC. Yet, the emergence of chemoresistance results in therapeutic failure and significant relapse despite a consistent rate of primary response. Emerging evidence substantiates the potential role of lncRNAs in determining the response to standard chemotherapy in OC. The objective of this narrative review is to provide an integrated, synthesized overview of the current state of knowledge regarding the role of lncRNAs in the emergence of resistance to platinum- and taxane-based chemotherapy in OC. In addition, we sought to develop conceptual frameworks for harnessing the therapeutic potential of lncRNAs in strategies aimed at enhancing the chemotherapy response of OC. Furthermore, we offered significant new perspectives and insights on the interplay between lncRNAs and the molecular circuitries implicated in chemoresistance to determine their impacts on therapeutic response. Although this review summarizes robust data concerning the involvement of lncRNAs in the emergence of acquired resistance to platinum- and taxane-based chemotherapy in OC, effective approaches for translating these lncRNAs into clinical practice warrant further investigation.
Keywords: chemotherapy; dysregulated expression; long non-coding RNAs; mechanisms of resistance; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ducie J., Dao F., Considine M., Olvera N., Shaw P.A., Kurman R.J., Shih I.-M., Soslow R.A., Cope L., Levine D.A. Molecular analysis of high-grade serous ovarian carcinoma with and without associated serous tubal intra-epithelial carcinoma. Nat. Commun. 2017;8:1–9. doi: 10.1038/s41467-017-01217-9. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

