Aza-BODIPY: A New Vector for Enhanced Theranostic Boron Neutron Capture Therapy Applications
- PMID: 32854219
- PMCID: PMC7565158
- DOI: 10.3390/cells9091953
Aza-BODIPY: A New Vector for Enhanced Theranostic Boron Neutron Capture Therapy Applications
Abstract
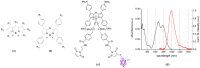
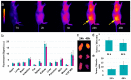
Boron neutron capture therapy (BNCT) is a radiotherapeutic modality based on the nuclear capture of slow neutrons by stable 10B atoms followed by charged particle emission that inducing extensive damage on a very localized level (<10 μm). To be efficient, a sufficient amount of 10B should accumulate in the tumor area while being almost cleared from the normal surroundings. A water-soluble aza-boron-dipyrromethene dyes (BODIPY) fluorophore was reported to strongly accumulate in the tumor area with high and BNCT compatible Tumor/Healthy Tissue ratios. The clinically used 10B-BSH (sodium borocaptate) was coupled to the water-soluble aza-BODIPY platform for enhanced 10B-BSH tumor vectorization. We demonstrated a strong uptake of the compound in tumor cells and determined its biodistribution in mice-bearing tumors. A model of chorioallantoic membrane-bearing glioblastoma xenograft was developed to evidence the BNCT potential of such compound, by subjecting it to slow neutrons. We demonstrated the tumor accumulation of the compound in real-time using optical imaging and ex vivo using elemental imaging based on laser-induced breakdown spectroscopy. The tumor growth was significantly reduced as compared to BNCT with 10B-BSH. Altogether, the fluorescent aza-BODIPY/10B-BSH compound is able to vectorize and image the 10B-BSH in the tumor area, increasing its theranostic potential for efficient approach of BNCT.
Keywords: 10B-BSH; BNCT; NIR-I; SWIR; aza-BODIPY; boron compound; in ovo model; optical imaging; theranostic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

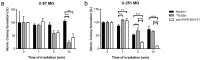

References
-
- Locher G. Biological effects and therapeutic possibilities of neutrons. Am. J. Roentgenol. Radium Ther. 1936;36:1–13.
-
- Aiyama H., Nakai K., Yamamoto T., Nariai T., Kumada H., Ishikawa E., Isobe T., Endo K., Takada T., Yoshida F., et al. A clinical trial protocol for second line treatment of malignant brain tumors with BNCT at University of Tsukuba. Appl. Radiat. Isot. 2011;69:1819–1822. doi: 10.1016/j.apradiso.2011.04.031. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

