Detection of Ovarian Cancer through Exhaled Breath by Electronic Nose: A Prospective Study
- PMID: 32854242
- PMCID: PMC7565069
- DOI: 10.3390/cancers12092408
Detection of Ovarian Cancer through Exhaled Breath by Electronic Nose: A Prospective Study
Abstract
Background: Diagnostic methods for the early identification of ovarian cancer (OC) represent an unmet clinical need, as no reliable diagnostic tools are available. Here, we tested the feasibility of electronic nose (e-nose), composed of ten metal oxide semiconductor (MOS) sensors, as a diagnostic tool for OC detection.
Methods: Women with suspected ovarian masses and healthy subjects had volatile organic compounds analysis of the exhaled breath using e-nose.
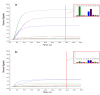
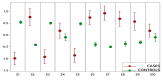
Results: E-nose analysis was performed on breath samples collected from 251 women divided into three groups: 86 OC cases, 51 benign masses, and 114 controls. Data collected were analyzed by Principal Component Analysis (PCA) and K-Nearest Neighbors' algorithm (K-NN). A first 1-K-NN (cases vs. controls) model has been developed to discriminate between OC cases and controls; the model performance tested in the prediction gave 98% of sensitivity and 95% of specificity, when the strict class prediction was applied; a second 1-K-NN (cases vs. controls + benign) model was built by grouping the non-cancer groups (controls + benign), thus considering two classes, cases and controls + benign; the model performance in the prediction was of 89% for sensitivity and 86% for specificity when the strict class prediction was applied.
Conclusions: Our preliminary results suggested the potential role of e-nose for the detection of OC. Further studies aiming to test the potential adoption of e-nose in the early diagnosis of OC are needed.
Keywords: K-NN models; MOS sensors; early diagnosis; electronic nose; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- FDA The FDA Recommends against Using Screening Tests for Ovarian Cancer Screening: FDA Safety Communication. [(accessed on 11 August 2020)];2016 Available online: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm519413.htm.

