CRISPR/Cas9 Delivery Potentials in Alzheimer's Disease Management: A Mini Review
- PMID: 32854251
- PMCID: PMC7559557
- DOI: 10.3390/pharmaceutics12090801
CRISPR/Cas9 Delivery Potentials in Alzheimer's Disease Management: A Mini Review
Abstract
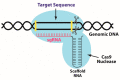
Alzheimer's disease (AD) is the most common dementia disorder. While genetic mutations account for only 1% of AD cases, sporadic AD resulting from a combination of genetic and risk factors constitutes >90% of the cases. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-associated protein (Cas9) is an impactful gene editing tool which identifies a targeted gene sequence, creating a double-stranded break followed by gene inactivation or correction. Although CRISPR/Cas9 can be utilized to irreversibly inactivate or correct faulty genes in AD, a safe and effective delivery system stands as a challenge against the translation of CRISPR therapeutics from bench to bedside. While viral vectors are efficient in CRISPR/Cas9 delivery, they might introduce fatal side effects and immune responses. As non-viral vectors offer a better safety profile, cost-effectiveness and versatility, they can be promising for the in vivo delivery of CRISPR/Cas9 therapeutics. In this minireview, we present an overview of viral and non-viral vector based CRISPR/Cas9 therapeutic strategies that are being evaluated on pre-clinical AD models. Other promising non-viral vectors that can be used for genome editing in AD, such as nanoparticles, nanoclews and microvesicles, are also discussed. Finally, we list the formulation and technical aspects that must be considered in order to develop a successful non-viral CRISPR/Cas9 delivery vehicle.
Keywords: Alzheimer’s disease; CRISPR; drug delivery; gene editing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gaugler J., James B., Johnson T., Marin A., Weuve J. Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15:321–387.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

