Lamin A/C and the Immune System: One Intermediate Filament, Many Faces
- PMID: 32854281
- PMCID: PMC7504305
- DOI: 10.3390/ijms21176109
Lamin A/C and the Immune System: One Intermediate Filament, Many Faces
Abstract
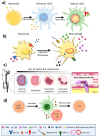
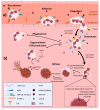
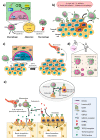
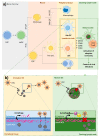
Nuclear envelope lamin A/C proteins are a major component of the mammalian nuclear lamina, a dense fibrous protein meshwork located in the nuclear interior. Lamin A/C proteins regulate nuclear mechanics and structure and control cellular signaling, gene transcription, epigenetic regulation, cell cycle progression, cell differentiation, and cell migration. The immune system is composed of the innate and adaptive branches. Innate immunity is mediated by myeloid cells such as neutrophils, macrophages, and dendritic cells. These cells produce a rapid and nonspecific response through phagocytosis, cytokine production, and complement activation, as well as activating adaptive immunity. Specific adaptive immunity is activated by antigen presentation by antigen presenting cells (APCs) and the cytokine microenvironment, and is mainly mediated by the cellular functions of T cells and the production of antibodies by B cells. Unlike most cell types, immune cells regulate their lamin A/C protein expression relatively rapidly to exert their functions, with expression increasing in macrophages, reducing in neutrophils, and increasing transiently in T cells. In this review, we discuss and summarize studies that have addressed the role played by lamin A/C in the functions of innate and adaptive immune cells in the context of human inflammatory and autoimmune diseases, pathogen infections, and cancer.
Keywords: Leishmania; T cell; cancer; dendritic cell (DC); inflammatory bowel disease; lamin A/C; macrophage; neutrophil; viral infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

