Diurnal Variation of Urinary Fabry Disease Biomarkers during Enzyme Replacement Therapy Cycles
- PMID: 32854306
- PMCID: PMC7503492
- DOI: 10.3390/ijms21176114
Diurnal Variation of Urinary Fabry Disease Biomarkers during Enzyme Replacement Therapy Cycles
Abstract
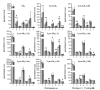
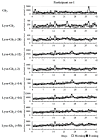
Fabry disease is an X-linked lysosomal storage disorder caused by mutations in the GLA gene encoding the α-galactosidase A enzyme. This enzyme cleaves the last sugar unit of glycosphingolipids, including globotriaosylceramide (Gb3), globotriaosylsphingosine (lyso-Gb3), and galabiosylceramide (Ga2). Enzyme impairment leads to substrate accumulation in different organs, vascular endothelia, and biological fluids. Enzyme replacement therapy (ERT) is a commonly used treatment. Urinary analysis of Gb3 isoforms (different fatty acid moieties), as well as lyso-Gb3 and its analogues, is a reliable way to monitor treatment. These analogues correspond to lyso-Gb3 with chemical modifications on the sphingosine moiety (-C2H4, -C2H4+O, -H2, -H2+O, +O, +H2O2, and +H2O3). The effects of sample collection time on urinary biomarker levels between ERT cycles were not previously documented. The main objective of this project was to analyze the aforementioned biomarkers in urine samples from seven Fabry disease patients (three treated males, three treated females, and one ERT-naïve male) collected twice a day (morning and evening) for 42 days (three ERT cycles). Except for one participant, our results show that the biomarker levels were generally more elevated in the evening. However, there was less variability in samples collected in the morning. No cyclic variations in biomarker levels were observed between ERT infusions.
Keywords: Fabry disease; diurnal variation; globotriaosylceramide; globotriaosylsphingosine; glycosphingolipids; mass spectrometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Michaud L., Auray-Blais C. Improved ways to screen for patients with fabry disease, involving optometry in a multidisciplinary approach. Can. J. Optom. 2012;74:25–32. doi: 10.15353/cjo.74.550. - DOI
-
- Lenders M., Stappers F., Niemietz C., Schmitz B., Boutin M., Ballmaier P.J., Zibert A., Schmidt H., Brand S.M., Auray-Blais C., et al. Mutation-specific fabry disease patient-derived cell model to evaluate the amenability to chaperone therapy. J. Med. Genet. 2019;56:548–556. doi: 10.1136/jmedgenet-2019-106005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

