Molecular Pharmacology of Synthetic Cannabinoids: Delineating CB1 Receptor-Mediated Cell Signaling
- PMID: 32854313
- PMCID: PMC7503917
- DOI: 10.3390/ijms21176115
Molecular Pharmacology of Synthetic Cannabinoids: Delineating CB1 Receptor-Mediated Cell Signaling
Abstract
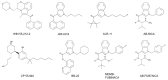
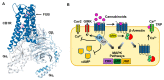
Synthetic cannabinoids (SCs) are a class of new psychoactive substances (NPSs) that exhibit high affinity binding to the cannabinoid CB1 and CB2 receptors and display a pharmacological profile similar to the phytocannabinoid (-)-trans-Δ9-tetrahydrocannabinol (THC). SCs are marketed under brand names such as K2 and Spice and are popular drugs of abuse among male teenagers and young adults. Since their introduction in the early 2000s, SCs have grown in number and evolved in structural diversity to evade forensic detection and drug scheduling. In addition to their desirable euphoric and antinociceptive effects, SCs can cause severe toxicity including seizures, respiratory depression, cardiac arrhythmias, stroke and psychosis. Binding of SCs to the CB1 receptor, expressed in the central and peripheral nervous systems, stimulates pertussis toxin-sensitive G proteins (Gi/Go) resulting in the inhibition of adenylyl cyclase, a decreased opening of N-type Ca2+ channels and the activation of G protein-gated inward rectifier (GIRK) channels. This combination of signaling effects dampens neuronal activity in both CNS excitatory and inhibitory pathways by decreasing action potential formation and neurotransmitter release. Despite this knowledge, the relationship between the chemical structure of the SCs and their CB1 receptor-mediated molecular actions is not well understood. In addition, the potency and efficacy of newer SC structural groups has not been determined. To address these limitations, various cell-based assay technologies are being utilized to develop structure versus activity relationships (SAR) for the SCs and to explore the effects of these compounds on noncannabinoid receptor targets. This review focuses on describing and evaluating these assays and summarizes our current knowledge of SC molecular pharmacology.
Keywords: CB1 receptors; cell signaling assays; molecular pharmacology; synthetic cannabinoids.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Molecular signaling of synthetic cannabinoids: Comparison of CB1 receptor and TRPV1 channel activation.Eur J Pharmacol. 2021 Sep 15;907:174301. doi: 10.1016/j.ejphar.2021.174301. Epub 2021 Jul 2. Eur J Pharmacol. 2021. PMID: 34224700 Free PMC article.
-
Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications.Drug Alcohol Depend. 2014 Nov 1;144:12-41. doi: 10.1016/j.drugalcdep.2014.08.005. Epub 2014 Aug 18. Drug Alcohol Depend. 2014. PMID: 25220897 Free PMC article. Review.
-
Application of an activity-based receptor bioassay to investigate the in vitro activity of selected indole- and indazole-3-carboxamide-based synthetic cannabinoids at CB1 and CB2 receptors.Drug Test Anal. 2019 Mar;11(3):501-511. doi: 10.1002/dta.2517. Epub 2018 Nov 18. Drug Test Anal. 2019. PMID: 30280499
-
Cannabinoid-mediated elevation of intracellular calcium: a structure-activity relationship.J Pharmacol Exp Ther. 2006 May;317(2):820-9. doi: 10.1124/jpet.105.100503. Epub 2006 Jan 25. J Pharmacol Exp Ther. 2006. PMID: 16436496
-
Spicing Up Pharmacology: A Review of Synthetic Cannabinoids From Structure to Adverse Events.Adv Pharmacol. 2017;80:135-168. doi: 10.1016/bs.apha.2017.05.001. Epub 2017 Jun 20. Adv Pharmacol. 2017. PMID: 28826533 Review.
Cited by
-
The synthetic cannabinoid 5F-MDMB-PICA enhances the metabolic activity and angiogenesis in human brain microvascular endothelial cells by upregulation of VEGF, ANG-1, and ANG-2.Toxicol Res (Camb). 2023 Aug 23;12(5):796-806. doi: 10.1093/toxres/tfad068. eCollection 2023 Oct. Toxicol Res (Camb). 2023. PMID: 37915478 Free PMC article.
-
Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery.Int J Mol Sci. 2022 Oct 30;23(21):13223. doi: 10.3390/ijms232113223. Int J Mol Sci. 2022. PMID: 36362014 Free PMC article. Review.
-
Synthetic Cannabinoids and Cathinones Cardiotoxicity: Facts and Perspectives.Curr Neuropharmacol. 2021;19(11):2038-2048. doi: 10.2174/1570159X19666210412101929. Curr Neuropharmacol. 2021. PMID: 33845747 Free PMC article.
-
Neuroprotection of retinal ganglion cells in vivo using the activation of the endogenous cannabinoid signaling system in mammalian eyes.Neuronal Signal. 2022 Feb 16;6(1):NS20210038. doi: 10.1042/NS20210038. eCollection 2022 Apr. Neuronal Signal. 2022. PMID: 35233292 Free PMC article.
-
Synaptic changes induced by cannabinoid drugs and cannabis use disorder.Neurobiol Dis. 2022 Jun 1;167:105670. doi: 10.1016/j.nbd.2022.105670. Epub 2022 Feb 24. Neurobiol Dis. 2022. PMID: 35219856 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

