Formation and Maturation of the Phagosome: A Key Mechanism in Innate Immunity against Intracellular Bacterial Infection
- PMID: 32854338
- PMCID: PMC7564318
- DOI: 10.3390/microorganisms8091298
Formation and Maturation of the Phagosome: A Key Mechanism in Innate Immunity against Intracellular Bacterial Infection
Abstract
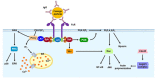
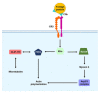
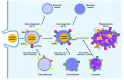
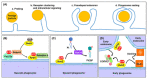
Phagocytosis is an essential mechanism in innate immune defense, and in maintaining homeostasis to eliminate apoptotic cells or microbes, such as Mycobacterium tuberculosis, Salmonella enterica, Streptococcus pyogenes and Legionella pneumophila. After internalizing microbial pathogens via phagocytosis, phagosomes undergo a series of 'maturation' steps, to form an increasingly acidified compartment and subsequently fuse with the lysosome to develop into phagolysosomes and effectively eliminate the invading pathogens. Through this mechanism, phagocytes, including macrophages, neutrophils and dendritic cells, are involved in the processing of microbial pathogens and antigen presentation to T cells to initiate adaptive immune responses. Therefore, phagocytosis plays a role in the bridge between innate and adaptive immunity. However, intracellular bacteria have evolved diverse strategies to survive and replicate within hosts. In this review, we describe the sequential stages in the phagocytosis process. We also discuss the immune evasion strategies used by pathogens to regulate phagosome maturation during intracellular bacterial infection, and indicate that these might be used for the development of potential therapeutic strategies for infectious diseases.
Keywords: bacterial infection; innate defense mechanism; innate immunity; lysosome; pathogen; phagocytosis; phagosome maturation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

