Recent Advances in Microbial Production of cis,cis-Muconic Acid
- PMID: 32854378
- PMCID: PMC7564838
- DOI: 10.3390/biom10091238
Recent Advances in Microbial Production of cis,cis-Muconic Acid
Abstract
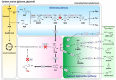
cis,cis-Muconic acid (MA) is a valuable C6 dicarboxylic acid platform chemical that is used as a starting material for the production of various valuable polymers and drugs, including adipic acid and terephthalic acid. As an alternative to traditional chemical processes, bio-based MA production has progressed to the establishment of de novo MA pathways in several microorganisms, such as Escherichia coli, Corynebacterium glutamicum, Pseudomonas putida, and Saccharomyces cerevisiae. Redesign of the metabolic pathway, intermediate flux control, and culture process optimization were all pursued to maximize the microbial MA production yield. Recently, MA production from biomass, such as the aromatic polymer lignin, has also attracted attention from researchers focusing on microbes that are tolerant to aromatic compounds. This paper summarizes recent microbial MA production strategies that involve engineering the metabolic pathway genes as well as the heterologous expression of some foreign genes involved in MA biosynthesis. Microbial MA production will continue to play a vital role in the field of bio-refineries and a feasible way to complement various petrochemical-based chemical processes.
Keywords: cis,cis-Muconic acid; metabolic engineering; microbial production; shikimate pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Muconic Acid Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2019–2024. [(accessed on 1 June 2019)]; Available online: https://www.researchandmarkets.com/reports/4775781/muconic-acid-market-g....
-
- Draths K.M., Frost J.W. Environmentally compatible synthesis of adipic acid from D-glucose. J. Am. Chem. Soc. 1994;116:399–400. doi: 10.1021/ja00080a057. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

