Current Clinical Trials Protocols and the Global Effort for Immunization against SARS-CoV-2
- PMID: 32854391
- PMCID: PMC7564421
- DOI: 10.3390/vaccines8030474
Current Clinical Trials Protocols and the Global Effort for Immunization against SARS-CoV-2
Abstract
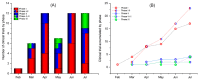
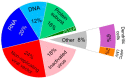
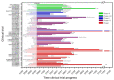
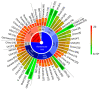
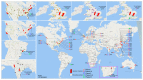
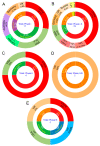
Coronavirus disease 2019 (COVID-19) is the biggest health challenge of the 21st century, affecting millions of people globally. The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has ignited an unprecedented effort from the scientific community in the development of new vaccines on different platforms due to the absence of a broad and effective treatment for COVID-19 or prevention strategy for SARS-CoV-2 dissemination. Based on 50 current studies selected from the main clinical trial databases, this systematic review summarizes the global race for vaccine development against COVID-19. For each study, the main intervention characteristics, the design used, and the local or global center partnerships created are highlighted. Most vaccine developments have taken place in Asia, using a viral vector method. Two purified inactivated SARS-CoV-2 vaccine candidates, an mRNA-based vaccine mRNA1273, and the chimpanzee adenoviral vaccine ChAdOx1 are currently in phase III clinical trials in the respective countries Brazil, the United Arab Emirates, the USA, and the United Kingdom. These vaccines are being developed based on a quickly formed network of collaboration.
Keywords: COVID-19; D); SARS-CoV-2; immunization; research and development (R& vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Worldometer COVID-19 Pandemic Data Update. [(accessed on 27 July 2020)];2020 Available online: https://www.worldometers.info/coronavirus/
-
- Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31605-6. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

