HLA Expression Correlates to the Risk of Immune Checkpoint Inhibitor-Induced Pneumonitis
- PMID: 32854442
- PMCID: PMC7564884
- DOI: 10.3390/cells9091964
HLA Expression Correlates to the Risk of Immune Checkpoint Inhibitor-Induced Pneumonitis
Abstract
Tumor-infiltrating T cell rescue by programmed cell death receptor-1 (PD-1)/PD-1 ligand-1 (PD-L1) immune checkpoint blockade is a recommended treatment for malignant diseases, including metastatic non-small-cell lung cancer (mNSCLC), malignant melanoma (MM), head and neck, kidney, and urothelial cancer. Monoclonal antibodies (mAbs) against either PD-1 or PD-L1 are active agents for these patients; however, their use may be complicated by unpredictable immune-related adverse events (irAEs), including immune-related pneumonitis (IRP). We carried out a retrospective multi-institutional statistical analysis to investigate clinical and biological parameters correlated with IRP rate on a cohort of 256 patients who received real-world treatment with PD-1/PD-L1 blocking mAbs. An independent radiological review board detected IRP in 29 patients. We did not find statistical IRP rate correlation with gender, tumor type, specific PD-1 or PD-L1 blocking mAbs, radiation therapy, inflammatory profile, or different irAEs. A higher IRP risk was detected only in mNSCLC patients who received metronomic chemotherapy +/- bevacizumab compared with other treatments prior PD-1/PD-L1 blockade. Moreover, we detected a strong correlation among the IRP rate and germinal expression of HLA-B*35 and DRB1*11, alleles associated to autoimmune diseases. Our findings may have relevant implications in predicting the IRP rate in mNSCLC patients receiving PD-1/PD-L1 blockade and need to be validated on a larger patient series.
Keywords: HLA-profile; PD-1/PD-L1 blockade; cancer immunotherapy; immune-related pneumonitis; irAEs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
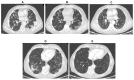
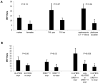
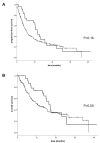
References
-
- Horn L., Spigel D.R., Vokes E.E., Holgado E., Ready N., Steins M., Poddubskaya E., Borghaei H., Felip E., Paz-Ares L., et al. Nivolumab versus docetaxel in previously treated patients with advanced Non-Small-Cell lung cancer: Two-Year outcomes from two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057) J. Clin. Oncol. 2017;35:3924–3933. doi: 10.1200/JCO.2017.74.3062. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

