Emerging Concepts of Motor Reserve in Parkinson's Disease
- PMID: 32854486
- PMCID: PMC7502292
- DOI: 10.14802/jmd.20029
Emerging Concepts of Motor Reserve in Parkinson's Disease
Abstract
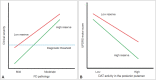
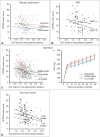
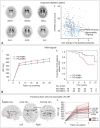
The concept of cognitive reserve (CR) in Alzheimer's disease (AD) explains the differences between individuals in their susceptibility to AD-related pathologies. An enhanced CR may lead to less cognitive deficits despite severe pathological lesions. Parkinson's disease (PD) is also a common neurodegenerative disease and is mainly characterized by motor dysfunction related to striatal dopaminergic depletion. The degree of motor deficits in PD is closely correlated to the degree of dopamine depletion; however, significant individual variations still exist. Therefore, we hypothesized that the presence of motor reserve (MR) in PD explains the individual differences in motor deficits despite similar levels of striatal dopamine depletion. Since 2015, we have performed a series of studies investigating MR in de novo patients with PD using the data of initial clinical presentation and dopamine transporter PET scan. In this review, we summarized the results of these published studies. In particular, some premorbid experiences (i.e., physical activity and education) and modifiable factors (i.e., body mass index and white matter hyperintensity on brain image studies) could modulate an individual's capacity to tolerate PD pathology, which can be maintained throughout disease progression.
Keywords: Dopamine transporter; Motor reserve; Parkinson’s disease; Positron-emission tomography.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



References
-
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. - PubMed
-
- Palmer SJ, Ng B, Abugharbieh R, Eigenraam L, McKeown MJ. Motor reserve and novel area recruitment: amplitude and spatial characteristics of compensation in Parkinson’s disease. Eur J Neurosci. 2009;29:2187–2196. - PubMed
LinkOut - more resources
Full Text Sources

