Treatment strategy and outcomes in locally advanced head and neck squamous cell carcinoma: a nationwide retrospective cohort study (KCSG HN13-01)
- PMID: 32854649
- PMCID: PMC7450571
- DOI: 10.1186/s12885-020-07297-z
Treatment strategy and outcomes in locally advanced head and neck squamous cell carcinoma: a nationwide retrospective cohort study (KCSG HN13-01)
Abstract
Background: By investigating treatment patterns and outcomes in locally advanced head and neck squamous cell carcinoma (LA-HNSCC), we aimed at providing valuable insights into the optimal therapeutic strategy for physicians in real-world practice.
Methods: This is a multi-institutional study enrolled the patients with stage III to IVB LA-HNSCC, except for nasopharyngeal carcinoma, from 2004 to 2015 in thirteen referral hospitals capable of multidisciplinary care.
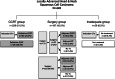
Results: A total of 445 LA-HNSCC patients were analyzed. The median age was 61 years (range, 24-89). The primary tumor location was the oropharynx in 191 (43%), oral cavity in 106 (24%), hypopharynx in 64 (14%), larynx in 57 (13%) and other sites in 27 (6%). The most common stage was T2 in 172 (39%), and N2 in 245 (55%). Based on treatment intents, 229 (52%) of the patients received definitive concurrent chemoradiotherapy (CCRT) and 187 (42%) underwent surgery. Approximately 158 (36%) of the study population received induction chemotherapy (IC). Taken together, 385 (87%) of the patients underwent combined therapeutic modalities. The regimen for definitive CCRT was weekly cisplatin in 58%, 3-weekly cisplatin in 28% and cetuximab in 3%. The preferred regimen for IC was docetaxel with cisplatin in 49%, and docetaxel, cisplatin plus fluorouracil in 27%. With a median follow-up of 39 months, one-year and two-year survival rates were 89 and 80%, respectively. Overall survival was not significantly different between CCRT and surgery group (p = 0.620).
Conclusions: In patients with LA-HNSCC, the majority of patients received combined therapeutic modalities. Definitive CCRT, IC then definitive CCRT, and surgery followed by adjuvant CCRT or radiotherapy are the preferred multidisciplinary strategies in real-world practice.
Keywords: Locally advanced head and neck cancer; Multidisciplinary treatment; Squamous cell carcinoma; Strategy.
Conflict of interest statement
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Figures

References
-
- Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB, Yunus F, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–2743. doi: 10.1200/JCO.2013.54.6309. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

