Synergistic interaction of hTGF-β3 with hBMP-6 promotes articular cartilage formation in chitosan scaffolds with hADSCs: implications for regenerative medicine
- PMID: 32854680
- PMCID: PMC7457281
- DOI: 10.1186/s12896-020-00641-y
Synergistic interaction of hTGF-β3 with hBMP-6 promotes articular cartilage formation in chitosan scaffolds with hADSCs: implications for regenerative medicine
Abstract
Background: Human TGF-β3 has been used in many studies to induce genes coding for typical cartilage matrix components and accelerate chondrogenic differentiation, making it the standard constituent in most cultivation media used for the assessment of chondrogenesis associated with various stem cell types on carrier matrices. However, in vivo data suggests that TGF-β3 and its other isoforms also induce endochondral and intramembranous osteogenesis in non-primate species to other mammals. Based on previously demonstrated improved articular cartilage induction by a using hTGF-β3 and hBMP-6 together on hADSC cultures and the interaction of TGF- β with matrix in vivo, the present study investigates the interaction of a chitosan scaffold as polyanionic polysaccharide with both growth factors. The study analyzes the difference between chondrogenic differentiation that leads to stable hyaline cartilage and the endochondral ossification route that ends in hypertrophy by extending the usual panel of investigated gene expression and stringent employment of quantitative PCR.
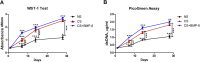
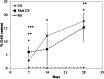
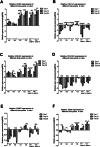
Results: By assessing the viability, proliferation, matrix formation and gene expression patterns it is shown that hTGF-β3 + hBMP-6 promotes improved hyaline articular cartilage formation in a chitosan scaffold in which ACAN with Col2A1 and not Col1A1 nor Col10A1 where highly expressed both at a transcriptional and translational level. Inversely, hTGF-β3 alone tended towards endochondral bone formation showing according protein and gene expression patterns.
Conclusion: These findings demonstrate that clinical therapies should consider using hTGF-β3 + hBMP-6 in articular cartilage regeneration therapies as the synergistic interaction of these morphogens seems to ensure and maintain proper hyaline articular cartilage matrix formation counteracting degeneration to fibrous tissue or ossification. These effects are produced by interaction of the growth factors with the polysaccharide matrix.
Keywords: Adipose-derived stem cell; Articular Chondrogenesis; Bone formation; Chitosan; Promotion; Synergism; Validation; hBMP-6; hTGF-β3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Hunter, W. On the structure and diseases of articulating cartilage. Phil Trans R Soc A 1743, 42B, 514–521.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

