Clinical significance of chromatin-spliceosome acute myeloid leukemia: a report from the Northern Italy Leukemia Group (NILG) randomized trial 02/06
- PMID: 32855275
- PMCID: PMC8485674
- DOI: 10.3324/haematol.2020.252825
Clinical significance of chromatin-spliceosome acute myeloid leukemia: a report from the Northern Italy Leukemia Group (NILG) randomized trial 02/06
Abstract
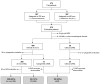
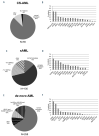
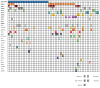
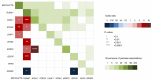
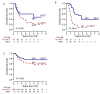
Secondary acute myeloid leukemia (sAML) after myelodysplastic or myeloproliferative disorders is a high-risk category currently identified by clinical history or specific morphological and cytogenetic abnormalities. However, in the absence of these features, uncertainties remain to identify the secondary nature of some cases otherwise defined as de novo AML. To test whether a chromatin-spliceosome (CS) mutational signature might better inform the definition of the de novo AML group, we analyzed a prospective cohort of 413 newly diagnosed AML patients enrolled into a randomized clinical trial (NILG AML 02/06) and provided with accurate cytogenetic and molecular characterization. Among clinically defined de novo AML, 17.6% carried CS mutations (CS-AML) and showed clinical characteristics closer to sAML (older age, lower white blood cell counts and higher rate of multilineage dysplasia). Outcomes in this group were adverse, more similar to those of sAML as compared to de novo AML (overall survival, 30% in CS-AML and 17% in sAML vs 61% in de novo AML, P<0.0001; disease free survival, 26% in CS-AML and 22% in sAML vs 54% of de novo AML, P<0.001) and independently confirmed by multivariable analysis. Allogeneic transplant in first complete remission improved survival in both sAML and CS-AML patients. In conclusion, these findings highlight the clinical significance of identifying CS-AML for improved prognostic prediction and potential therapeutic implications. (NILG AML 02/06: ClinicalTrials.gov Identifier: NCT00495287).
Figures

References
-
- Arber DA, Orazi A, Hasserjian R, et al. . The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. - PubMed
-
- Weinberg OK, Seetharam M, Ren L, et al. . Clinical characterization of acute myeloid leukemia with myel-odysplasia-related changes as defined by the 2008 WHO classification system. Blood. 2009;113(9):1906-1908. - PubMed

