Fetal hemoglobin rescues ineffective erythropoiesis in sickle cell disease
- PMID: 32855279
- PMCID: PMC8485663
- DOI: 10.3324/haematol.2020.265462
Fetal hemoglobin rescues ineffective erythropoiesis in sickle cell disease
Abstract
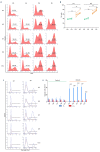
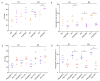
While ineffective erythropoiesis has long been recognized as a key contributor to anemia in thalassemia, its role in anemia of sickle cell disease (SCD) has not been critically explored. Using in vitro and in vivo derived human erythroblasts we assessed the extent of ineffective erythropoiesis in SCD. Modeling the bone marrow hypoxic environment, we found that hypoxia induces death of sickle erythroblasts starting at the polychromatic stage, positively selecting cells with high levels of fetal hemoglobin (HbF). Cell death was associated with cytoplasmic sequestration of heat shock protein 70 and was rescued by induction of HbF synthesis. Importantly, we document that in the bone marrow of SCD patients similar cell loss occurs during the final stages of terminal differentiation. Our study provides evidence for ineffective erythropoiesis in SCD and highlights an anti-apoptotic role for HbF during the terminal stages of erythroid differentiation. These findings imply that the beneficial effect on anemia of increased HbF levels is not only due to the increased life span of red cells but also a consequence of decreased ineffective erythropoiesis.
Figures




References
-
- Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science. 1949;110(3):543-548. - PubMed
-
- Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561-1573. - PubMed
-
- Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311-323. - PubMed
-
- Arlet JB, Dussiot M, Moura IC, Hermine O, Courtois G.Novel players in beta-thalassemia dyserythropoiesis and new therapeutic strategies. Curr Opin Hematol. 2016;23(3):181-188. - PubMed
-
- Arlet JB, Ribeil JA, Guillem F, et al. . HSP70 sequestration by free alpha-globin promotes ineffective erythropoiesis in beta-thalassaemia. Nature. 2014;514(7521):242-246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

